Product: MIL-101(Cr)
Synonyms: NA
CAS:869288-09-5
Basic Information
| Unit MF. | C24H12O15FCr3 | Unit MW. | 715.33 | ||
| Coordination Metal | Cr | Linkers | Terephthalic acid (CAS:100-21-0) | ||
| Aperture |
Aperture:1.2-1.6nm; Pore Size: 2.9-3.4nm |
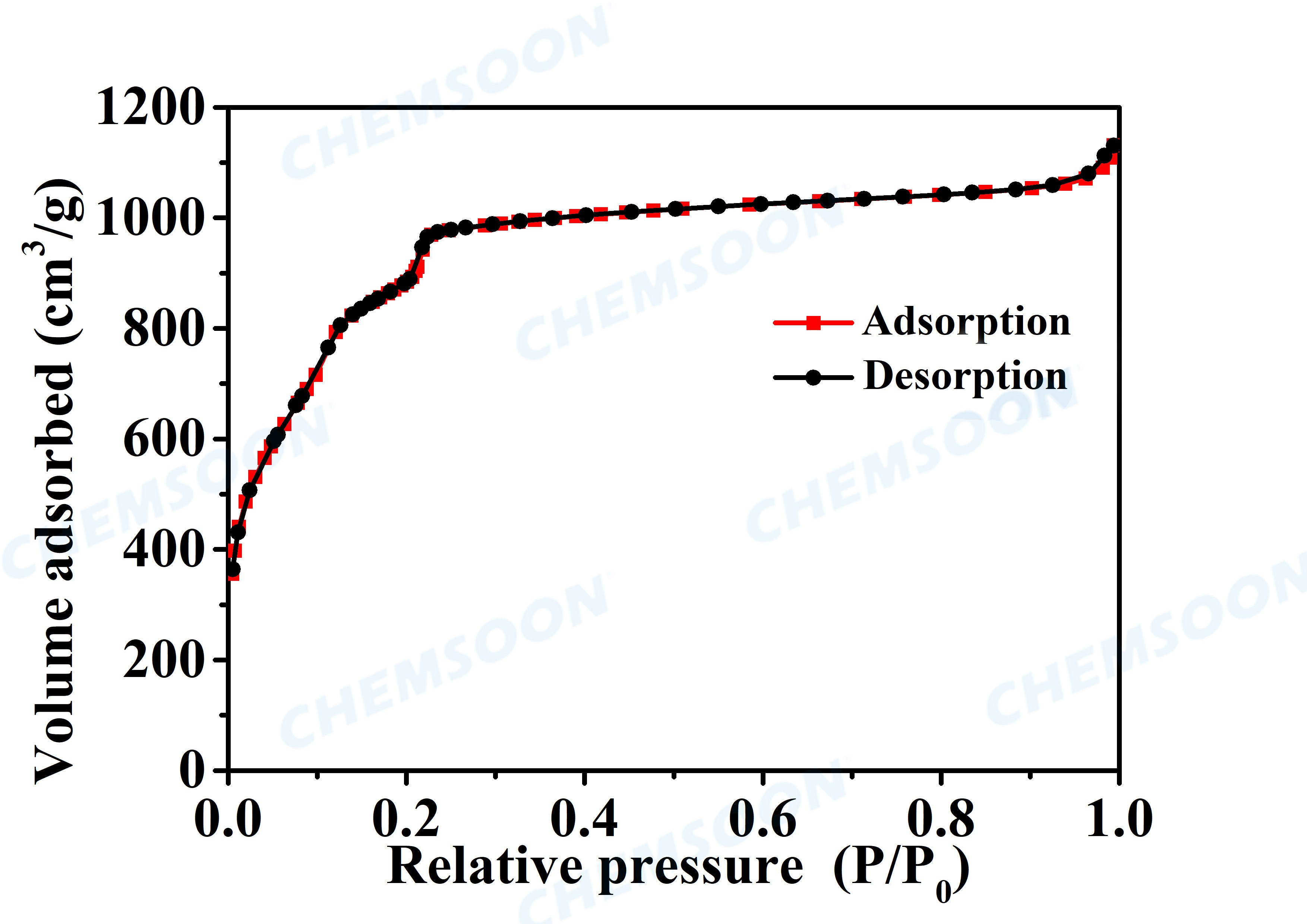
Pore volume | 2.0-2.4 cm3/g | ||
| Surface Area | BET Specific surface 2800-3200 m2/g, | ||||
| Analog Structure |  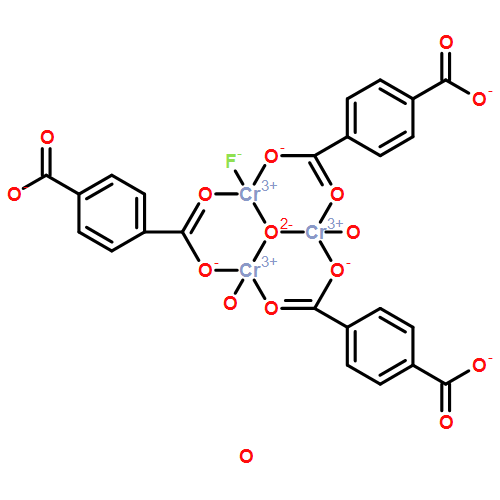 |
||||
Product Property
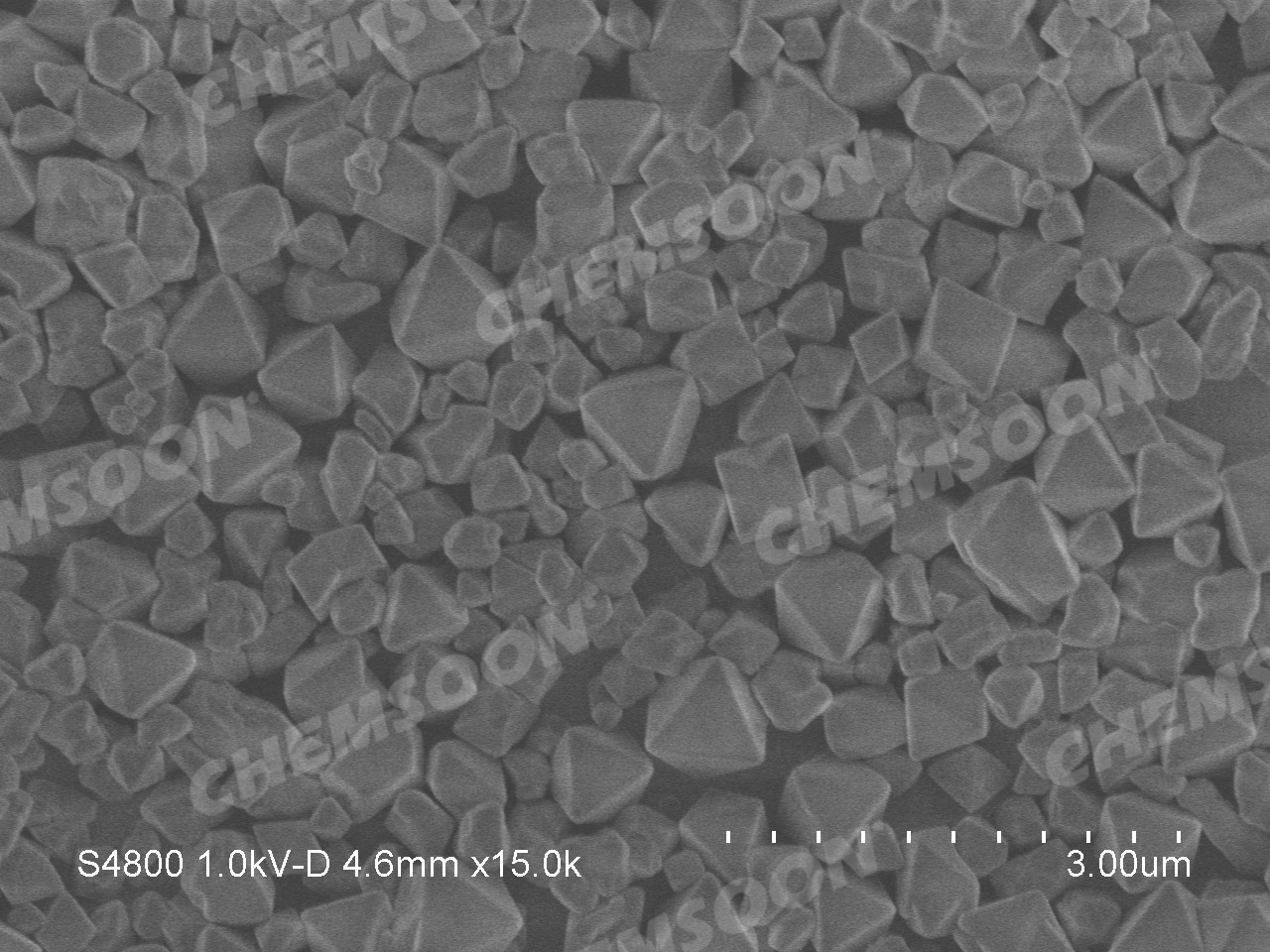
| Appearance | Grey Green Powder |  |
||
| Particle Size |
500nm-1um |
|||
Stability
1) MIL-101(Cr) is stable in air and acqeous solutions (PH 1-12)
2)Thermal stability, thermal decomposition temperature above 300 ° C
2)Thermal stability, thermal decomposition temperature above 300 ° C
Preservation
1) Keep sealed in dry and cool condition
2) It is recommended to activate for 10 hours at 110 degree in vacuum before gas-adsorption test.
Other Features
Fluorescence:NA
Applications
1) Gas (such as carbon dioxide) and pollutant adsorption
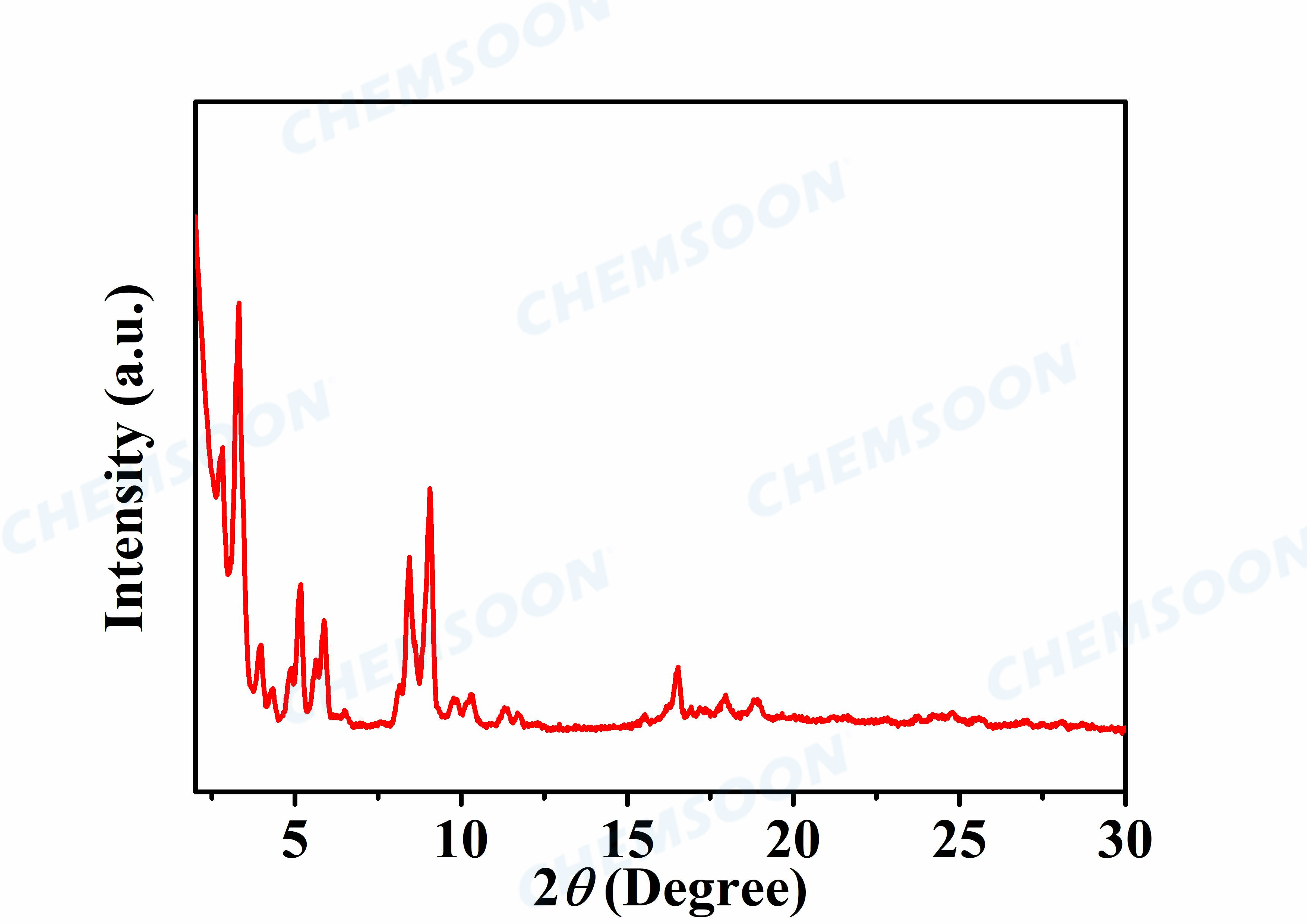
Characterizations
References
1) G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé, I. Margiolaki, Science 2005, 309(5743), 2040-2042, DOI: 10.1126/science.1116275 ; A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area;
2)Lomig Hamon, Christian Serre, Thomas Devic, Thierry Loiseau, Franck Millange, Gérard Férey, Guy De Weireld; Journal of the American Chemical Society, 2009, 131, 25; DOI:10.1021/ja901587t; Comparative Study of Hydrogen Sulfide Adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) Metal-Organic Frameworks at Room Temperature.;
3)Young Kyu Hwang, Do-Young Hong, Jong-San Chang, Sung Hwa Jhung, You-Kyong Seo, Jinheung Kim, Alexandre Vimont, Marco Daturi, Christian Serre, Gerard Ferey; Angewandte Chemie International Edition, 2008, 47, 4144-4148; DOI:10.1002/anie.200705998; Amine Grafting on Coordinatively Unsaturated Metal Centers of MOFs: Consequences for Catalysis and Metal Encapsulation;
4)Philip L. Llewellyn, Sandrine Bourrelly, Christian Serre, Alexandre Vimont, Marco Daturi, Lomig Hamon, Guy De Weireld, Jong-San Chang, Do-Young Hong, Young Kyu Hwang, Sung Hwa Jhung, Gérard Ferey; Langmuir, 2008, 24, 14, 7245-7250; DOI:10.1021/la800227x; High Uptakes of CO₂ and CH₄ in Mesoporous Metal-Organic Frameworks MIL-100 and MIL-101;
5)Sara Sorribas, Patricia Gorgojo, Carlos Tellez, Joaquín Coronas, Andrew G. Livingston; Journal of the American Chemical Society, 2013, 135, 15201-15208; DOI:10.1021/ja407665w; High Flux Thin Film Nanocomposite Membranes Based on Metal−Organic Frameworks for Organic Solvent Nanofiltration;
6)Enamul Haque, Ji Eun Lee, In Tae Jang, Young Kyu Hwang, Jong-San Chang, Jonggeon Jegal, Sung Hwa Jhung; Journal of Hazardous Materials, 2010, 181, 535-542; DOI:10.1016/j.jhazmat.2010.05.047; Adsorptive removal of methyl orange from aqueous solution with metal-organic frameworks, porous chromium-benzenedicarboxylates;
7)Sung Hwa Jhung, Jin-Ho Lee, Ji Woong Yoon, Christian Serre, Gérard Férey, Jong-San Chang*; Advanced Materials, 2007, 19, 121-124; DOI:10.1002/adma.200601604; Microwave Synthesis of Chromium Terephthalate MIL‐101 and Its Benzene Sorption Ability;
8)Lomig Hamon, Christian Serre, Thomas Devic, Thierry Loiseau, Franck Millange, Gérard Férey, Guy De Weireld;Journal of the American Chemical Society, 2009, Volume 131, Issue 25;DOI:10.1021/ja901587t;Comparative Study of Hydrogen Sulfide Adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) Metal-Organic Frameworks at Room Temperature;
9)Cheng-Xiong Yang and Xiu-Ping Yan*;Analytical Chemistry, 2011, 83, 7144–7150; DOI:10.1021/ac201517c; Metal-Organic Framework MIL-101(Cr) for High-Performance Liquid Chromatographic Separation of Substituted Aromatics