Home >
News > Comparative study on the adsorption performance of metal organic framework materials for hydrogen sulfide at room temperature
Comparative study on the adsorption performance of metal organic framework materials for hydrogen sulfide at room temperature
Summary:
The authors from Laboratoire de Thermodynamique (Belgium) and Institut Lavoisier (France) studied 7 types of Metal-Organic Frameworks (MOFs) with different rigidity/flexibility and pore sizes, achieving results in H₂S adsorption and natural gas purification.
Background:
1. To address the problem of removing H₂S (a pollutant in natural gas) and the deficiencies of existing adsorbents (e.g., chemisorption leading to high regeneration costs, poor stability), previous researchers used amine absorption for bulk sulfur removal and porous solids for deep purification, yet most porous solids have limited suitability for H₂S adsorption.
2. The authors proposed an innovative approach: selecting 7 MOFs (MIL-53 series, MIL-47(V), MIL-100(Cr), MIL-101(Cr)) to study their H₂S adsorption performance, and confirmed that some MOFs (e.g., MIL-47(V), MIL-53(Al, Cr)) are stable, regenerable, and highly selective for H₂S.
Research Content:
1. Synthesis
The authors synthesized MIL-47(V), MIL-53(Al, Cr, Fe), MIL-100(Cr), and MIL-101(Cr) following previous reports; MIL-100(Cr) and MIL-101(Cr) used as-synthesized solids, while others were activated by outgassing (393.1 K for most, 473.1 K for MIL-47(V)).
2. Characterizations
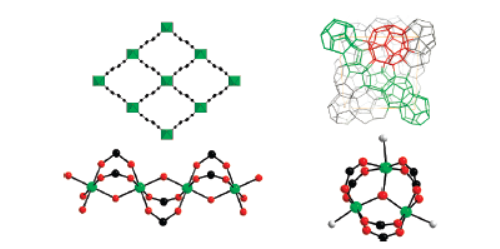
1)BET and pore size: MIL-53(Al, Cr, V) and MIL-47(V) have ~1000 m²/g BET surface area and 1D diamond-shaped pores; MIL-100(Cr) and MIL-101(Cr) have BET surface areas over 2000 m²/g (2600 m²/g for MIL-101(Cr), 1900 m²/g for MIL-100(Cr)) and mesopores.
2)SEM/TEM tests: No specific particle size data from SEM/TEM tests was provided in the articles.
3)Other tests: High-pressure magnetic suspension balance measured H₂S/CH₄ adsorption isotherms (303.1 K, up to 2.0 MPa); desorption experiments and BET surface area measurements after H₂S treatment evaluated stability/regeneration.
3. Application
-H₂S adsorption: At 303.1 K and 2.0 MPa, MIL-101(Cr) had the highest H₂S adsorption capacity (38.4 mmol/g), followed by MIL-100(Cr) (16.7 mmol/g), MIL-47(V) (14.6 mmol/g), MIL-53(Cr) (13.12 mmol/g), MIL-53(Al) (11.77 mmol/g), and MIL-53(Fe) (8.53 mmol/g).
-Natural gas purification: MIL-47(V) showed ~30 H₂S/CH₄ selectivity at 1.0 MPa; MIL-53(Al, Cr) had reversible adsorption, suitable for pressure swing adsorption (PSA) with 6 mmol/g dynamic capacity.
-Regeneration: MIL-47(V) and MIL-53(Al, Cr) recovered initial weight/CH₄ adsorption capacity after 8 h vacuum treatment at 393.1 K; MIL-101(Cr)/MIL-100(Cr) had partial irreversibility.
4. Mechanism
-Adsorption mechanism: Rigid MOFs (MIL-47(V), MIL-100(Cr), MIL-101(Cr)) showed type-I isotherms (monotonic adsorption); flexible MIL-53 series had two-step isotherms (pore closure at low H₂S loading via H₂S-OH interactions, reopening at high pressure).
-Simulation mechanism: Vacancy Solution Theory (VST) simulated H₂S-CH₄ binary adsorption on MIL-47(V); though VST underestimated low-pressure H₂S adsorption, it confirmed high H₂S selectivity, supporting experimental results.
Outlook:
This research is the first experimental study on H₂S adsorption in the 7 MOFs, confirming that MIL-47(V) and MIL-53(Al, Cr) are stable, regenerable, and highly selective for H₂S. It provides a basis for MOFs’ application in natural gas purification and guides the design of H₂S adsorbents.
Comparative Study of Hydrogen Sulfide Adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) Metal-Organic Frameworks at Room Temperature
Authors: Lomig Hamon, Christian Serre, Thomas Devic, Thierry Loiseau, Franck Millange, Gérard Férey, Guy De Weireld
DOI: 10.1021/ja901587t
Link: https://pubs.acs.org/doi/10.1021/ja901587t
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

