Home >
News > Amine-grafted MIL-101 (ED/APS/DETA) with CUSs shows high activity in Knoevenagel/Heck catalysis.
Amine-grafted MIL-101 (ED/APS/DETA) with CUSs shows high activity in Knoevenagel/Heck catalysis.
Summary:
The authors from organizations including Korea Research Institute of Chemical Technology (KRICT), Kyungpook National University, Ewha Womans University, CNRS/ENSICAEN et Université de Caen Basse-Normandie, and Institut Lavoisier developed amine-grafted MIL-101 (ED-MIL-101, APS-MIL-101, DETA-MIL-101) and noble metal-encapsulated amine-MIL-101 with high thermal stability and size-selective catalysis, achieving excellent performance in Knoevenagel condensation and Heck coupling reaction fields.
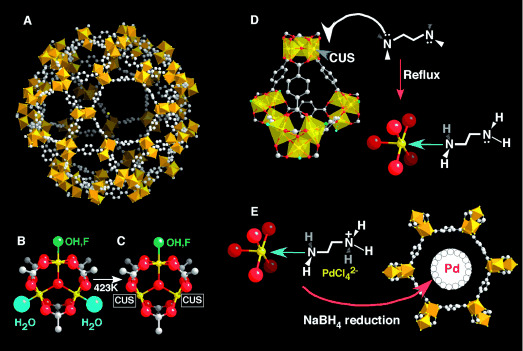
Background:
1. To address the need for efficient surface functionalization of porous materials for catalysis and metal encapsulation, previous researchers functionalized mesoporous silicas with amines (requiring surface hydroxyl groups) and modified MOFs via ligand functionalization or primary synthesis of low-coordination metal centers. However, these methods either lack unsaturated surface sites (mesoporous silicas) or are limited to ligand modification (traditional MOF methods), failing to realize direct and selective functionalization of metal sites.
2. The authors proposed a new strategy: using coordinatively unsaturated metal sites (CUSs) of MIL-101 to selectively graft amines, realizing direct coordination of electron-rich groups to CUSs. This method avoids reliance on surface hydroxyl groups, and the resulting amine-grafted MIL-101 exhibits high catalytic activity and enables effective noble metal encapsulation.
Research Content:
1. Synthesis
(1)MIL-101 Synthesis and Purification
- Hydrothermal synthesis: Terephthalic acid, Cr(NO₃)₃·9H₂O, HF, and deionized water were reacted at 493 K for 8 h to obtain as-synthesized MIL-101 (containing unreacted terephthalic acid).
- Purification: First, double filtration (40-100 μm glass filters) removed free terephthalic acid; then solvothermal treatment with 95% ethanol at 353 K for 24 h; finally, soaking in 1 M NH₄F solution at 70°C for 24 h, followed by filtration, hot water washing, and drying at 423 K.
(2)Amine Grafting on MIL-101
- General method: 0.5 g dehydrated MIL-101 (423 K, 12 h under vacuum) was suspended in 30 mL anhydrous toluene; 0.75 mmol of amine (ED/APS/DETA) was added, and the mixture was refluxed for 12 h.
- Special case: ED-MIL-101(D) (double ED grafting) was prepared by adding 1.5 mmol ED, with other conditions unchanged.
- Post-treatment: The product was filtered, washed with deionized water/ethanol, and dried at room temperature.
(3)Noble Metal Encapsulation
- 1.0 g ED-MIL-101 was mixed with aqueous solutions of PdCl₂, H₂PtCl₆, or HAuCl₄ (1.0 wt%, pH≈3) for 6 h to form M-NH₃⁺-MIL-101 via electrostatic interaction.
- Reduction: The solid was filtered, washed, and reduced with 0.07 g NaBH₄ in 10 mL ethanol at 273 K for 2 h to obtain Pd/ED-MIL-101, Pt/ED-MIL-101, and Au/ED-MIL-101 (metal loading ~0.93-0.96 wt% via ICP).
2. Characterizations
(1)BET and Pore Size Distribution
- As-synthesized MIL-101: BET surface area 4230 m²/g.
- Amine-grafted MIL-101: BET surface area decreased (e.g., ED-MIL-101 and DETA-MIL-101 showed significant drops); BJH pore size distribution indicated slight pore size reduction due to amine grafting in mesopore cages.
- SBA-15 (for comparison): BET surface area 780 m²/g.
(2)SEM/TEM Tests
- SEM: The article did not mention specific particle size data of MIL-101 and amine-grafted MIL-101.
- TEM: Noble metal-encapsulated MIL-101 (e.g., Pd/ED-MIL-101) had 2-4 nm nanoparticles (matching MIL-101 cage diameter); Pd-impregnated MIL-101 (without amine grafting) formed larger particles (>10 nm).
(3)Other Tests
-XRD: XRD patterns of amine-grafted MIL-101 (ED/APS/DETA) were almost unchanged from pure MIL-101, confirming no loss of crystallinity; noble metal-encapsulated samples also retained MIL-101’s crystal structure.
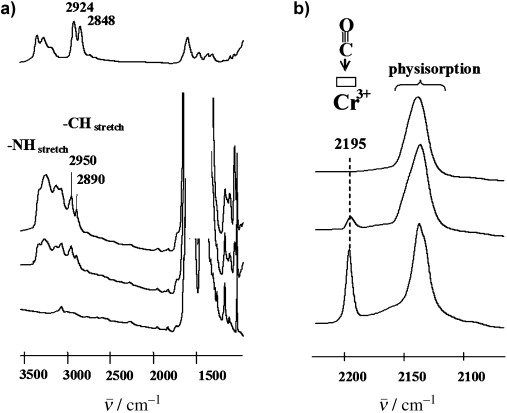
-FT-IR:
Amine grafting: ED-MIL-101 showed ν(NH) and ν(CH) bands (2800-3000 cm⁻¹, shifted due to coordination with Cr³⁺ CUSs); CO adsorption at 100 K showed decreased CUS concentration (disappeared in ED-MIL-101(D)).
Thermal stability: ED-MIL-101’s ν(CH) band remained stable up to 473 K, proving amine thermal stability.
-Elemental/TGA Analysis: ED-MIL-101 had ~1 ED molecule per Cr trimer, ED-MIL-101(D) had ~2; TGA confirmed amine content and thermal stability.
3. Application
(1)Knoevenagel Condensation (Benzaldehyde + Ethyl Cyanoacetate, 353 K)
- ED-MIL-101: 97.1% conversion, 99.1% selectivity (trans-ethyl cyanocinnamate), TOF 10x higher than APS-SBA-15.
- ED-MIL-101(D): Conversion similar to ED-MIL-101 (amine reactivity differences offset higher loading).
- APS-SBA-15: Only 74.8% conversion, 93.5% selectivity (lower due to H-bonding between APS groups).
- Size selectivity: ED-MIL-101 failed to catalyze benzophenone + malononitrile (large product occluded in pores).
- Recyclability: ED-MIL-101 retained activity after 3 cycles (no significant conversion drop).
(2)Heck Coupling Reaction (Iodobenzene + Acrylic Acid, 393 K)
- Pd/ED-MIL-101 (0.95 wt% Pd) and Pd/APS-MIL-101 (0.93 wt% Pd): Activity comparable to commercial Pd/C (1.09 wt% Pd) after 0.5-1 h induction period (slow reactant diffusion).
- Heterogeneity: Catalyst recyclable, no activity in filtrate (proving heterogeneous catalysis).
4. Mechanism
-Amine Grafting Mechanism: MIL-101’s Cr³⁺ CUSs (formed by removing terminal water at 423 K) act as Lewis acid sites, coordinating with amine’s electron-rich N atoms. This replaces residual terephthalic acid on CUSs, realizing selective grafting (confirmed by FT-IR CO adsorption and elemental analysis).
-Catalysis Mechanism:
Knoevenagel condensation: Grafted amine groups (free NH₂) act as base sites; MIL-101’s high surface area and accessible pores enhance reactant contact, leading to higher activity than APS-SBA-15 (avoiding H-bonding site loss).
Noble metal encapsulation: Amine’s NH₂ groups (protonated to NH₃⁺ in acidic conditions) interact electrostatically with anionic metal salts ([PdCl₄]²⁻, [PtCl₆]²⁻, [AuCl₄]⁻). Gentle NaBH₄ reduction forms small nanoparticles (2-4 nm) confined in MIL-101 cages, preventing agglomeration (unlike impregnated MIL-101’s large particles).
Outlook:
This research innovatively uses MOF CUSs for selective amine grafting, breaking the reliance of traditional porous material functionalization on surface hydroxyl groups. The amine-grafted MIL-101 exhibits excellent size-selective catalysis and thermal stability in Knoevenagel condensation, while the noble metal-encapsulated derivative performs well in Heck coupling. This strategy provides a universal method for MOF surface functionalization and metal encapsulation, expanding MOFs’ application in heterogeneous catalysis. Future work may explore grafting other functional groups to tailor catalytic properties for more reactions.
Amine Grafting on Coordinatively Unsaturated Metal Centers of MOFs: Consequences for Catalysis and Metal Encapsulation
Authors: Young Kyu Hwang, Do-Young Hong, Jong-San Chang, Sung Hwa Jhung, You-Kyong Seo, Jinheung Kim, Alexandre Vimont, Marco Daturi, Christian Serre, Gérard Férey
DOI: 10.1002/anie.200705998
Link: https://onlinelibrary.wiley.com/doi/10.1002/anie.200705998
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

