Home >
News > Comparative Study of Hydrogen Sulfide Adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) Metal-Organic Frameworks at Room Temperature
Comparative Study of Hydrogen Sulfide Adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) Metal-Organic Frameworks at Room Temperature
Summary:
The authors from Faculté Polytechnique de Mons and Université de Versailles Saint-Quentin-en-Yvelines studied several metal-organic frameworks (MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), MIL-101(Cr)) with characteristics like varying pore sizes (small to large) and rigidity/flexibility, achieving insights into their hydrogen sulfide (H₂S) adsorption performance for natural gas purification.
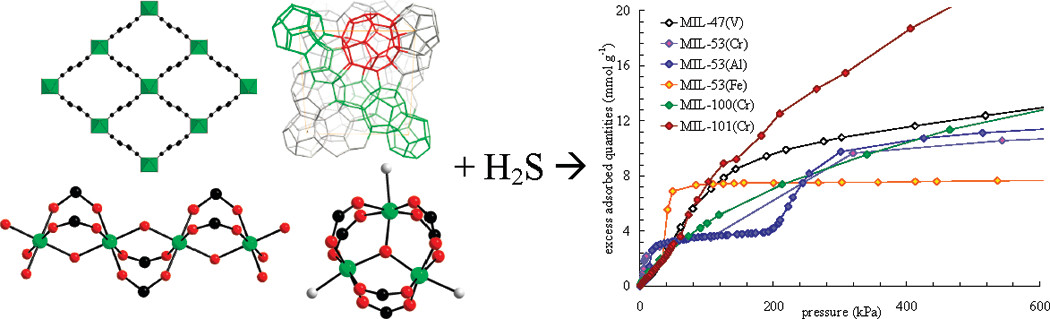
Background:
1. Natural gas purification requires removing sulfur compounds, with existing methods using amines or porous solids, but porous solids often have issues like chemisorption or high regeneration costs. MOFs have shown potential for gas adsorption but their H₂S adsorption properties were rarely studied.
2. The authors conducted a comparative study on H₂S adsorption in selected MOFs, examining isotherms, regeneration, and stability, finding some MOFs are stable and regenerable for H₂S sorption.
Research Content:
1. Synthesis:
The authors synthesized the materials using reported methods. MIL-47(V), MIL-53(Al, Cr, Fe), MIL-100(Cr), and MIL-101(Cr) were prepared and activated, with as-synthesized MIL-100 and MIL-101 used directly.
2. Characterizations:
1) BET and pore size distribution: MIL-100(Cr) and MIL-101(Cr) have large BET surface areas (>2000 m²/g); MIL-47(V) and MIL-53(Al, Cr) have ~1000 m²/g.
2) SEM/TEM tests: Not explicitly provided in the text.
3) Other tests: Adsorption isotherms measured via high-pressure magnetic suspension balance; H₂S and CH₄ adsorption capacities determined at 303.1 K.
3. Application:
The materials were tested for H₂S adsorption. Large-pore MIL-101(Cr) had maximum adsorption of 38.4 mmol/g at 2 MPa; MIL-53 series showed two-step isotherms due to flexibility. MIL-47(V) and MIL-53(Al, Cr) were regenerable, while MIL-53(Fe) was destroyed. MIL-47(V) showed high H₂S/CH₄ selectivity (~30 at 1.0 MPa).
4. Mechanism:
MIL-100 and MIL-101 (large, rigid pores) show type-I isotherms. MIL-53 (flexible) exhibits two-step isotherms due to "breathing"—pore closure at low H₂S loading (strong interactions with OH groups) and reopening at high pressure. MIL-47(V) (rigid, no OH groups) shows type-I isotherms. Regeneration tests indicate physisorption in MIL-47(V) and MIL-53(Al, Cr), with partial irreversibility in MIL-100/101.
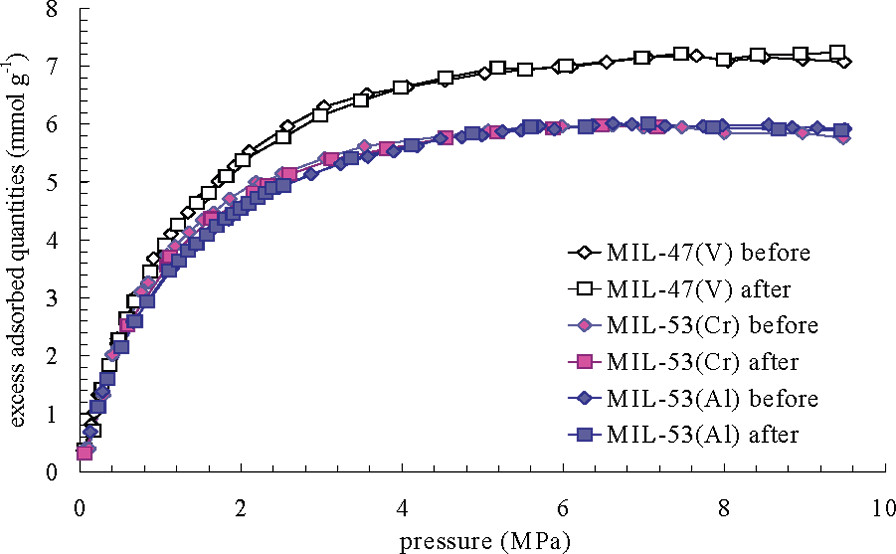
Outlook:
This research identifies stable and regenerable MOFs (MIL-47(V), MIL-53(Al, Cr)) for H₂S adsorption, showing potential for natural gas purification. Their unique adsorption behaviors (steps, selectivity) are promising for pressure swing adsorption processes.
Comparative Study of Hydrogen Sulfide Adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) Metal-Organic Frameworks at Room Temperature
Authors: Lomig Hamon, Christian Serre, Thomas Devic, Thierry Loiseau, Franck Millange, Gérard Férey, Guy De Weireld
DOI: 10.1021/ja901587t
Link: https://pubs.acs.org/doi/10.1021/ja901587t
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

