Home >
News > Metal-Organic Framework MIL-101(Cr) for High-Performance Liquid Chromatographic Separation of Substituted Aromatics
Metal-Organic Framework MIL-101(Cr) for High-Performance Liquid Chromatographic Separation of Substituted Aromatics
Summary:
The authors fromResearch Center for Analytical Sciences, College of Chemistry, and State Key Laboratory of Medicinal Chemical Biology, Nankai University, China developed the metal-organic framework MIL-101(Cr) with high surface area, large pore windows, mesoporous pores, and excellent chemical/solvent stability, achieving high-resolution separation of substituted aromatics in the application of high-performance liquid chromatography (HPLC) field.
Background:
1. To address the challenge of separating substituted aromatics (e.g., xylene, dichlorobenzene, chlorotoluene isomers, ethylbenzene (EB), styrene) due to their coherent boiling points and similar dimensions, previous researchers used MOFs like MIL-47, MIL-53(Al), MOF-5, and HKUST-1 as HPLC stationary phases, achieving partial separation success. Yet, most studies only used single pure solvents as mobile phases, limiting retention, selectivity, and resolution control.
2. The authors in this article proposed using a slurry-packed MIL-101(Cr) column as an HPLC stationary phase, obtained high column efficiency, good precision, and fast selective separation of ortho-isomers, and clarified the thermodynamic mechanisms (entropy/enthalpy control) of different separations.
Research Content:
1. Synthesis
The authors synthesized MIL-101(Cr) using a solvothermal method: mixing Cr(NO₃)₃·9H₂O (2.0 mmol), terephthalic acid (2.0 mmol), and HF (0.5 mmol) with ultrapure water (9.6 mL) in a Teflon-lined bomb, heating at 220°C for 8 h. The product was washed with DMF and ethanol (centrifugation at 10000 rpm for 5 min, repeated ≥3 times) and vacuum-evacuated at 150°C for 12 h to get dehydrated MIL-101(Cr).
2. Characterizations
1.Pore-related properties: MIL-101(Cr) has large pore windows (12 Å and 16 Å×14.5 Å) and mesoporous pores (29–34 Å), with high surface area (not quantified by BET in the text but noted as a key characteristic).
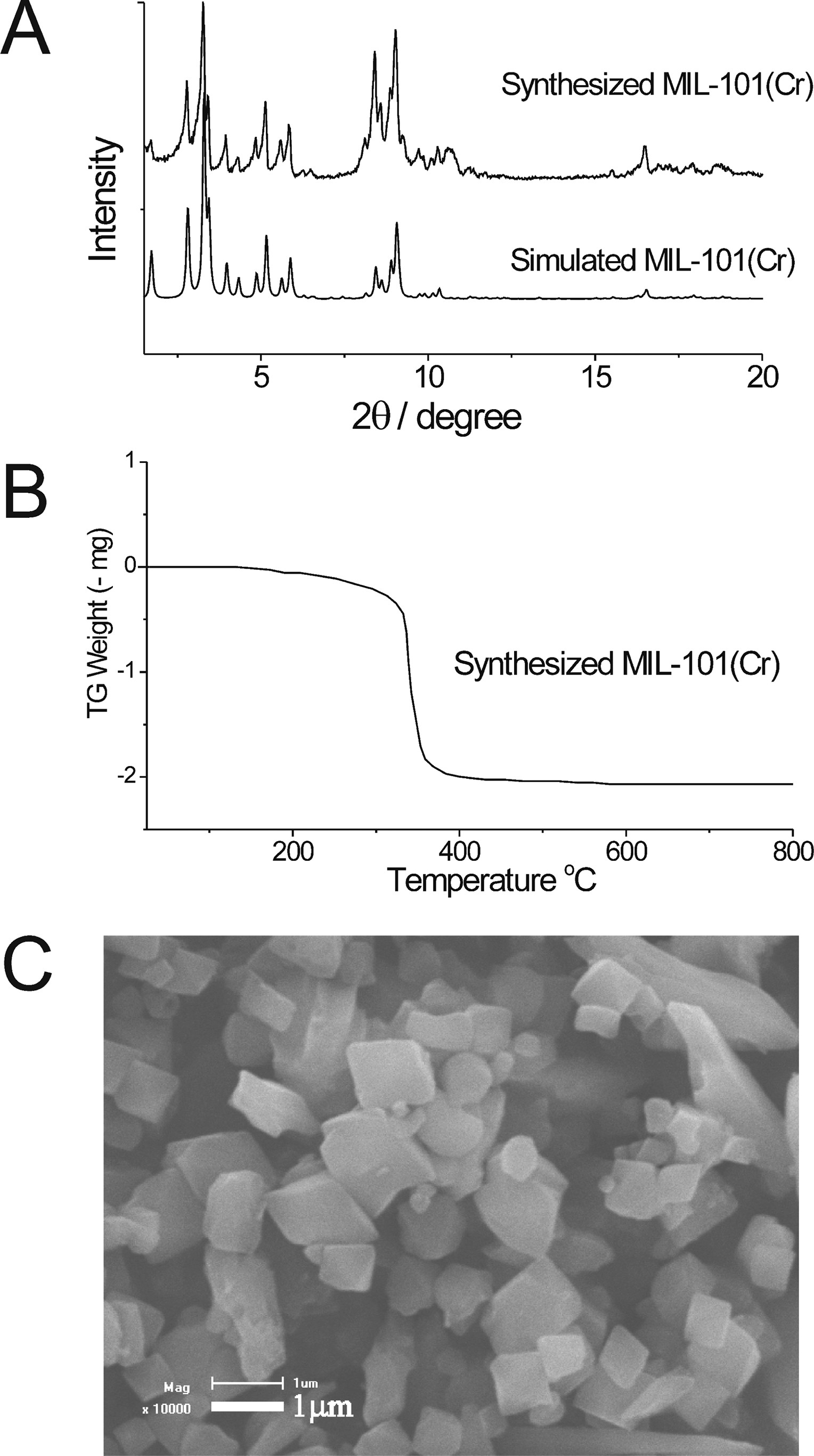
2.SEM test: The SEM image shows MIL-101(Cr) has cubic-shaped crystals with an average particle size of 1 μm.
3.Other tests:
- XRD: The experimental XRD pattern matches the simulated one, confirming successful synthesis.
- TGA: MIL-101(Cr) is stable up to 330°C.
3. Application
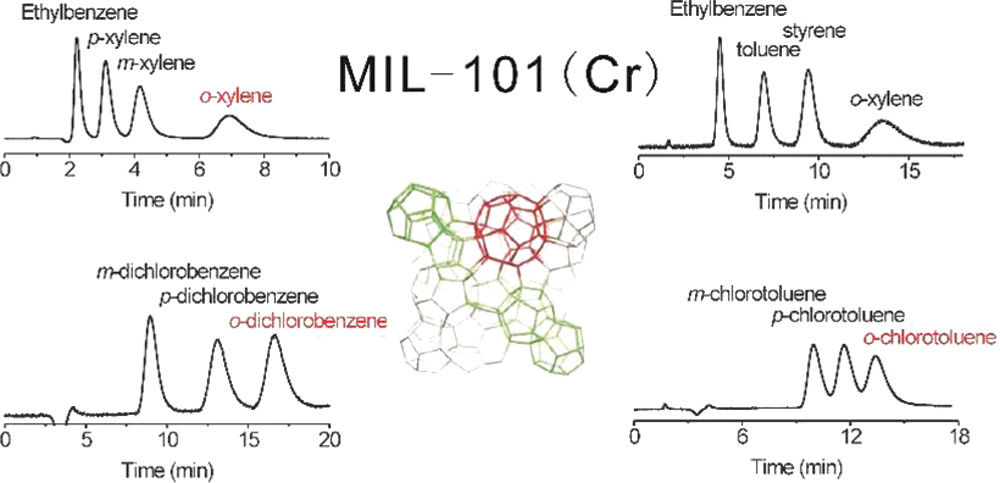
The material was tested as an HPLC stationary phase (5 cm long×4.6 mm i.d. slurry-packed column), with results as follows:
- Separated EB and xylene isomers (hexane/DCM 95:5 as mobile phase, 1 mL·min⁻¹ flow rate) with baseline separation, column efficiency of 20000 plates·m⁻¹ for EB, and RSD (n=5) of 0.3–0.4% (retention time), 1.5–2.9% (peak area), etc.
- Separated dichlorobenzene (hexane/DCM 90:10, 0.5 mL·min⁻¹) and chlorotoluene isomers (hexane/DCM 95:5, 0.5 mL·min⁻¹) with column efficiencies of 13000 plates·m⁻¹ (m-dichlorobenzene) and 10000 plates·m⁻¹ (m-chlorotoluene), respectively.
- Separated EB and styrene (hexane as mobile phase, 0.5 mL·min⁻¹) with a resolution of 3.4; also separated EB/styrene mixtures containing toluene and o-xylene impurities.
- Achieved fast selective separation of ortho-isomers within 3 min using pure DCM as mobile phase.
4. Mechanism
-Experimental result analysis: MIL-101(Cr) has high affinity for ortho-isomers due to their higher polarity, larger dimensions, and interactions with terephthalate pore walls; styrene retention is enhanced by π-π interaction with MIL-101(Cr)’s aromatic framework and coordinative interaction with unsaturated Cr sites.
-Thermodynamic calculation: Using the van’t Hoff equation, separation of xylene, dichlorobenzene, and chlorotoluene isomers is controlled by entropy change (ΔS), while separation of EB and styrene is governed by enthalpy change (ΔH); all separations are exothermic (retention time decreases with temperature increase).
Outlook:
This research demonstrates MIL-101(Cr) as a promising HPLC stationary phase for substituted aromatics separation, overcoming challenges of similar boiling points/dimensions. It provides a basis for MOF applications in HPLC, emphasizing the value of MOFs’ permanent porosity, high surface area, and open metal sites. Future research could focus on MOFs for specific recognition and separation of large (including biological) molecules.
Metal-Organic Framework MIL-101(Cr) for High-Performance Liquid Chromatographic Separation of Substituted Aromatics
Authors: Cheng-Xiong Yang and Xiu-Ping Yan*
DOI: 10.1021/ac201517c
Link: https://pubs.acs.org/doi/10.1021/ac201517c
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

