Home >
News > Microwave Synthesis of Chromium Terephthalate MIL‐101
Microwave Synthesis of Chromium Terephthalate MIL‐101
Summary:
The authors fromKorea Research Institute of Chemical Technology (KRICT) andInstitut Lavoisier, UMR CNRS 8180, Université de Versailles Saint Quentin en Yvelines developed cubic chromium terephthalate MIL-101 with large pores, large surface area, and nanoscale size, achieving excellent benzene sorption performance in the application of harmful organic contaminant removal.
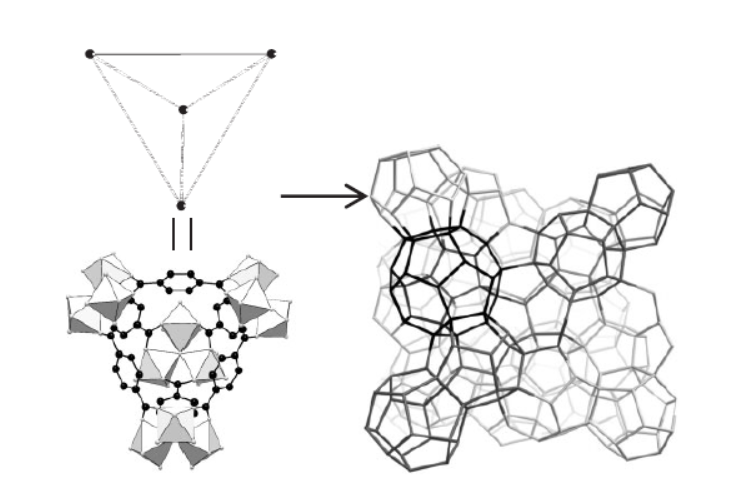
Background:
1. To address the demand for porous inorganic–organic hybrids with very large pores (for applications like gas storage, catalysis, and separations), previous researchers explored hydrothermal/solvothermal synthesis methods. However, these methods require several days of crystallization, which is inefficient for industrial-scale production.
2. The authors proposed an innovative microwave irradiation synthesis method, which significantly shortens the crystallization time of MIL-101 while maintaining its excellent physicochemical properties.
Research Content:
1. Synthesis
The authors synthesized cubic chromium terephthalate MIL-101 usingmicrowave irradiation (600 W) at 210 °C for 1–60 min. The reactant molar ratio was 1 Cr(NO₂)₃·9H₂O : 1 H₂BDC (benzene dicarboxylate) : 1 HF : 280 H₂O. The product was purified via double filtration (40–100 μm glass filters) and solvothermal treatment (95% ethanol, 100 °C, 20 h), followed by drying at 150 °C overnight.
2. Characterizations
1.BET and pore size distribution: For MIL-101(40, synthesized at 210 °C for 40 min), the BET surface area was 3900 m²g⁻¹, Langmuir surface area was 5900 ± 300 m²g⁻¹, total pore volume was 2.3 cm³g⁻¹, and BJH pore size distribution showed two monodisperse pores (13.0 Å and 18.2 Å).
2.SEM/TEM tests: MIL-101 crystals were nanoscale; MIL-101(1) had a size of 40–90 nm, MIL-101(2) 70–90 nm, and crystal size increased and became more homogeneous with longer crystallization time. These crystals were smaller than conventionally synthesized MIL-101(CH).
3.Other tests: XRD patterns matched the simulated MIL-101, confirming crystallinity; TGA showed stability up to 275 °C (weight loss in two steps: 25–200 °C for guest water loss, 275–400 °C for OH/F group departure and framework decomposition); FTIR detected O–C–O stretching at 1550 and 1430 cm⁻¹, confirming dicarboxylate groups.
3. Application
-Vapor-phase benzene sorption (30 °C): At P/P₀=0.5, MIL-101(40) had a capacity of 16.7 mmol g⁻¹, far higher than SBA-15 (3.0 mmol g⁻¹), HZSM-5 (1.9 mmol g⁻¹), and commercial active carbon (8.0 mmol g⁻¹); it reached adsorption equilibrium in 253 s, faster than MIL-101(CH) (300 s) and active carbon (500 s).
-Liquid-phase benzene sorption (25 °C): In 1000 ppm aqueous benzene solution, MIL-101(40) adsorbed more benzene than active carbon, with a faster sorption rate.
4. Mechanism
- Microwave irradiation accelerated synthesis via "hot spots" and superheating effects, promoting rapid precursor dissolution and metal–oxygen network condensation, shortening crystallization time.
- The fast benzene sorption of microwave-synthesized MIL-101 was attributed to its smaller crystal size and larger pore diameter, which reduced mass transfer resistance.
- The high benzene sorption capacity was due to its large surface area and suitable pore structure, enabling effective capture of benzene molecules.
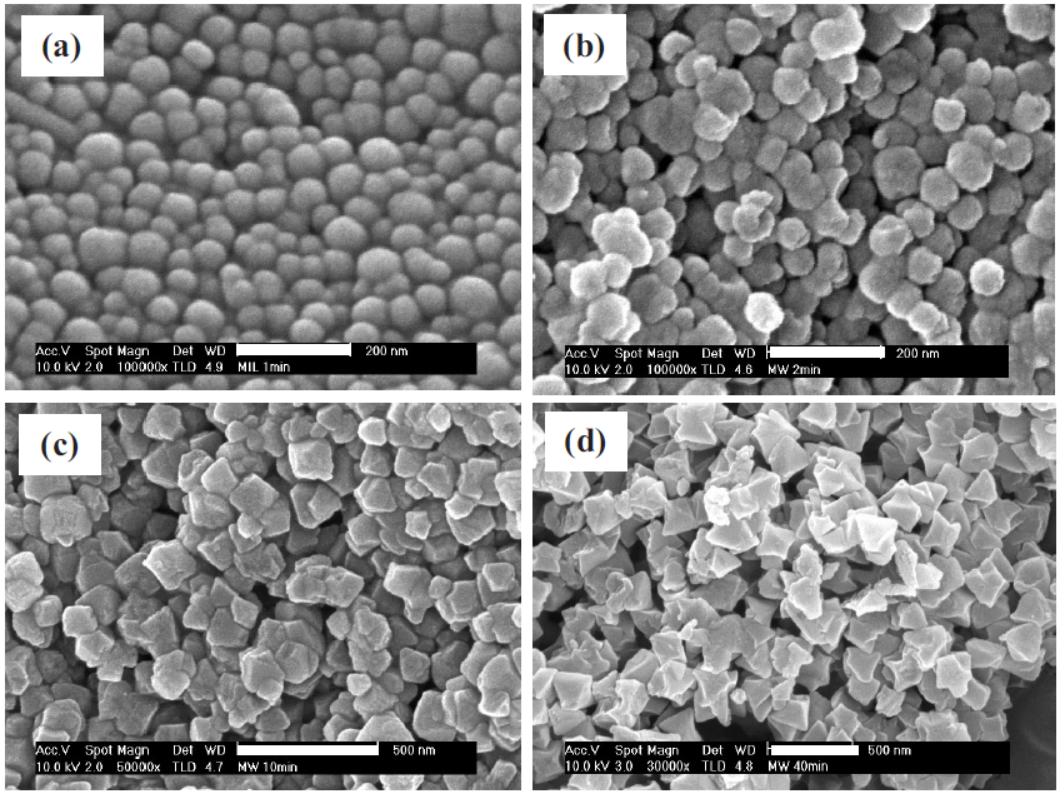
Outlook:
This research realizes the rapid, efficient synthesis of MIL-101 via microwave irradiation, overcoming the inefficiency of traditional hydrothermal methods. The synthesized MIL-101 has excellent benzene sorption performance, providing a new candidate for harmful organic contaminant removal. It also expands the application of microwave technology in porous hybrid material synthesis, laying a foundation for industrial production and related application development.
Microwave Synthesis of Chromium Terephthalate MIL‐101 and Its Benzene Sorption Ability
Authors: Sung Hwa Jhung, Jin-Ho Lee, Ji Woong Yoon, Christian Serre, Gérard Férey, Jong-San Chang
DOI: 10.1002/adma.200601604
Link: https://onlinelibrary.wiley.com/doi/10.1002/adma.200601604
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

