Home >
News > Adsorptive removal of methyl orange from aqueous solution with metal-organic frameworks, porous chromium-benzenedicarboxylates
Adsorptive removal of methyl orange from aqueous solution with metal-organic frameworks, porous chromium-benzenedicarboxylates
Summary:
The authors from Kyungpook National University and Korea Research Institute of Chemical Technology developed porous chromium-benzenedicarboxylate metal-organic frameworks (Cr-BDC MOFs: MIL-53, MIL-101, ED-MIL-101, PED-MIL-101) with high porosity and tunable surface charge, achieving efficient adsorptive removal of methyl orange (MO) from aqueous solutions.
Background:
1. To address the problem of toxic, carcinogenic, and难-degradable anionic dyes (e.g., MO) in industrial wastewater, previous researchers used adsorbents like ammonium-functionalized MCM-41, layered double hydroxides (LDH), and agricultural waste-derived activated carbon, achieving certain dye removal effects, yet there was a lack of research on metal-organic frameworks (MOFs) for this application.
2. The authors proposed using Cr-BDC MOFs (MIL-53, MIL-101) and modifying MIL-101 via ethylenediamine grafting (ED-MIL-101) and protonation (PED-MIL-101), obtaining adsorbents with high adsorption capacity and reusability for MO removal.
Research Content:
1.Synthesis
The authors synthesized MIL-53 and MIL-101 following reported methods; prepared ED-MIL-101 by grafting ethylenediamine onto MIL-101; obtained PED-MIL-101 by acidifying ED-MIL-101 with 0.1 M HCl at room temperature for 6 h; and purchased granular activated carbon (2–3 mm) as a reference.
2.Characterizations
1) BET: Activated carbon (1068 m²/g), MIL-53 (1438 m²/g), MIL-101 (3873 m²/g), ED-MIL-101 (3491 m²/g), PED-MIL-101 (3296 m²/g); total pore volumes: 0.50, 0.55, 1.70, 1.37, 1.18 cm³/g respectively; pore sizes: activated carbon/MIL-53 (<1.0 nm), MIL-101 (1.6, 2.1 nm), ED-MIL-101/PED-MIL-101 (1.4, 1.8 nm).
2) No SEM/TEM particle size data provided.
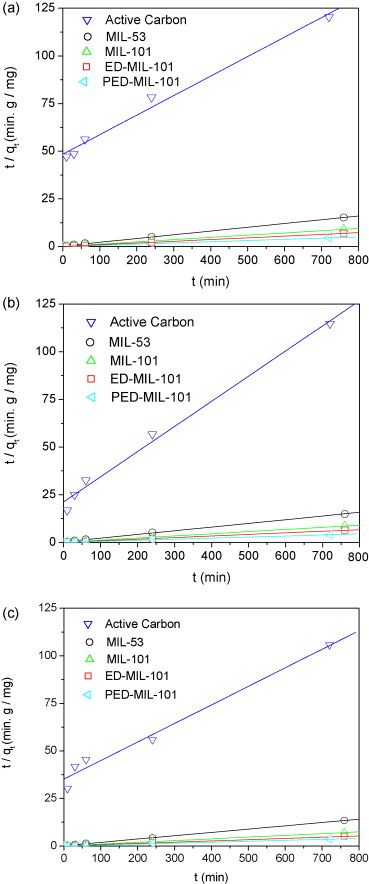
3) UV spectrophotometry (464 nm) quantified MO concentration; pseudo-first/second-order kinetics and Langmuir isotherms analyzed adsorption behavior.
3.Application
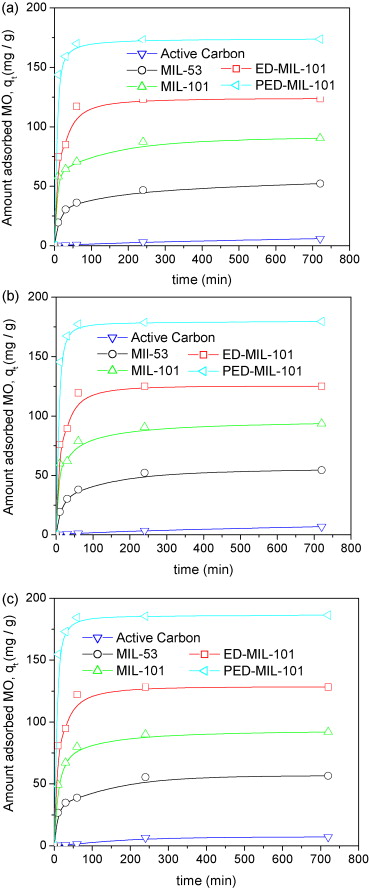
In MO adsorption: PED-MIL-101 had the highest capacity (194 mg/g at 25 °C), followed by ED-MIL-101 (160 mg/g), MIL-101 (114 mg/g), MIL-53 (57.9 mg/g), and activated carbon (11.2 mg/g); adsorption completed in 2 h for modified MIL-101s; after 2 reuses, PED-MIL-101’s qe remained ~181 mg/g.
4.Mechanism
- Electrostatic interaction: Anionic MO binds to cationic adsorbents (positive charge: MIL-101 < ED-MIL-101 < PED-MIL-101), so PED-MIL-101’s capacity decreases with increasing pH.
- Thermodynamics: PED-MIL-101’s ΔH=29.5 kJ/mol (endothermic), ΔS=208 J/(mol·K) (entropy increase), ΔG negative (spontaneous); entropy increase drives adsorption.
Outlook:
This research is the first to apply Cr-BDC MOFs for dye removal, proving modified MOFs (e.g., PED-MIL-101) are efficient, reusable adsorbents, providing a new direction for anionic dye wastewater treatment.
Adsorptive removal of methyl orange from aqueous solution with metal-organic frameworks, porous chromium-benzenedicarboxylates
Authors: Enamul Haque, Ji Eun Lee, In Tae Jang, Young Kyu Hwang, Jong-San Chang, Jonggeon Jegal, Sung Hwa Jhung
DOI: 10.1016/j.jhazmat.2010.05.047
Link: https://www.sciencedirect.com/science/article/pii/S0304389410006308
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

