Home >
News > Cr-based MOFs (MIL-100/MIL-101) with mesopores show high CO₂/CH₄ adsorption, synthesized via hydrothermal method.
Cr-based MOFs (MIL-100/MIL-101) with mesopores show high CO₂/CH₄ adsorption, synthesized via hydrothermal method.
Summary:
The authors from multiple organizations including Université de Provence - CNRS, Institut LaVoisier, CNRS/ENSICAEN et Université de Caen Basse-Normandie, Korea Research Institute of Chemical Technology (KRICT), and Faculté Polytechnique de Mons developed mesoporous metal-organic frameworks (MOFs) MIL-100(Cr) and MIL-101(Cr) with large pores and abundant Lewis acid sites, achieving record-high CO₂ uptake capacities in the application of greenhouse gas (CO₂ and CH₄) adsorption and separation field.
 Background:
1. To address the problem of high regeneration cost of traditional adsorbents (zeolites and activated carbons) for separating/storing greenhouse gases (CO₂ and CH₄), previous researchers developed porous MOFs with adjustable pores and mild regeneration conditions, yet their adsorption capacities and interaction strengths with target gases still had room for improvement.
2. The authors in this study proposed optimizing the synthesis and activation methods of MIL-100(Cr) and MIL-101(Cr), and obtained MIL-101c with a record CO₂ uptake capacity and confirmed the good regeneration performance of both MOFs.
Research Content:
1. Synthesis
- MIL-100(Cr): The authors synthesized it using a hydrothermal method: 0.5 g of chromium(VI) oxide, 1.05 g of trimesic acid, 1.0 mL of 5 M hydrofluoric acid solution, and 24 mL of deionized water were mixed, stirred at room temperature, then reacted in a Teflon-lined hydrothermal bomb at 493 K for 4 days; the product was washed and dried, with no additional activation needed.
- MIL-101(Cr): Three samples were prepared:
- MIL-101a (as-synthesized): Hydrothermally synthesized at 493 K for 8 h, followed by 3 h cooling to room temperature.
- MIL-101b: MIL-101a was activated with hot ethanol to remove unreacted terephthalic acid.
- MIL-101c: MIL-101b was treated in 150 mL of 30 mM F⁻ aqueous solution at 333 K for 10 h, then filtered and washed with hot water.
2. Characterizations
1. BET and pore size distribution:
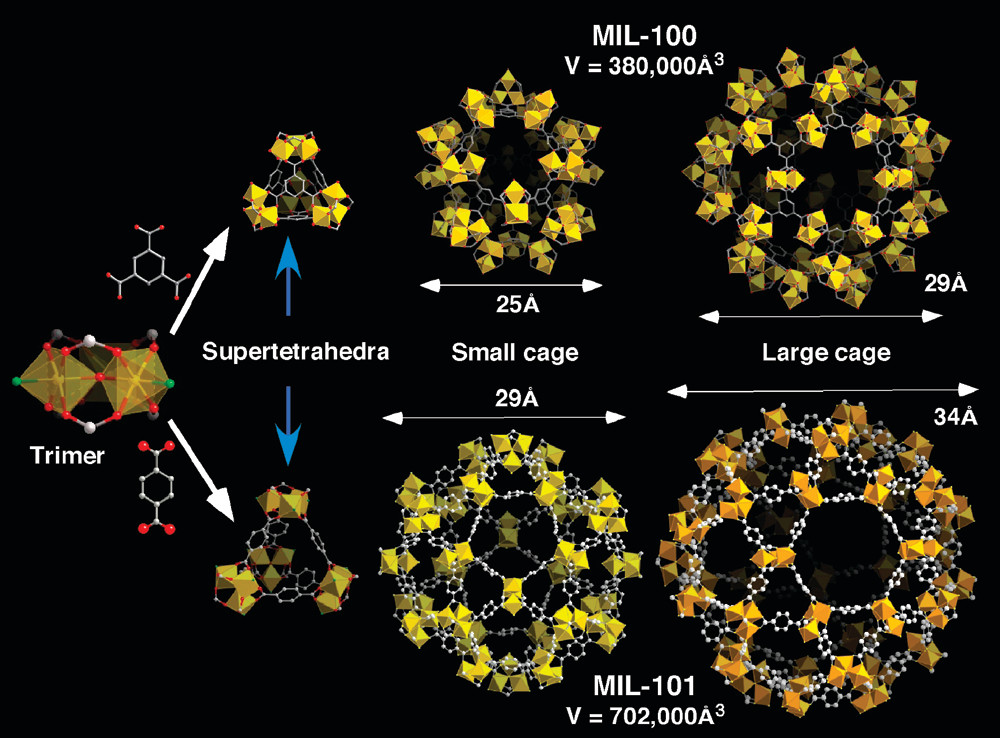
MIL-100: BET surface area 1900 m²/g, pore volume 1.10 cm³/g (at p/p⁰=0.8), free internal diameters of cages ~2.5 nm and 2.9 nm, window sizes 0.55 nm and 0.86 nm, cubic cell volume ~380000 ų.
MIL-101a: BET surface area 2800 m²/g, pore volume 1.37 cm³/g.
MIL-101b: BET surface area 3780 m²/g, pore volume 1.74 cm³/g.
MIL-101c: BET surface area 4230 m²/g, pore volume 2.15 cm³/g, free internal diameters of cages ~2.9 nm and 3.4 nm, window sizes 1.2 nm and 1.6 nm, cubic cell volume ~702000 ų.
2. SEM/TEM tests: The article did not mention SEM/TEM tests for particle size analysis.
3. Other tests:
Microcalorimetry: Enthalpy of CH₄ adsorption at zero coverage ~19 kJ/mol (MIL-100) and ~18 kJ/mol (MIL-101c); CO₂ adsorption enthalpy at zero coverage ~62 kJ/mol (MIL-100) and ~44 kJ/mol (MIL-101c).
FTIR: CO adsorption at 100 K showed MIL-100 had three ν(CO) bands (2207, 2200, 2193 cm⁻¹) for Lewis acid sites, MIL-101 had one ν(CO) band (2196 cm⁻¹); CO₂ adsorption showed ν₃ band (2348 cm⁻¹ for MIL-100, 2351 cm⁻¹ for MIL-101) and no carbonate species.
Manometry and gravimetry: Adsorption isotherms at 303 K confirmed reproducible gas uptake data across three labs.
3. Application
The materials were tested for CO₂ and CH₄ adsorption at 303 K:
- MIL-100: CH₄ uptake ~9.5 mmol/g at 3.5 MPa; CO₂ uptake 18 mmol/g (280 cm³/cm³) at 5 MPa.
- MIL-101:
- CH₄ uptake ~15 mmol/g at 6 MPa (135 cm³/cm³) for all samples.
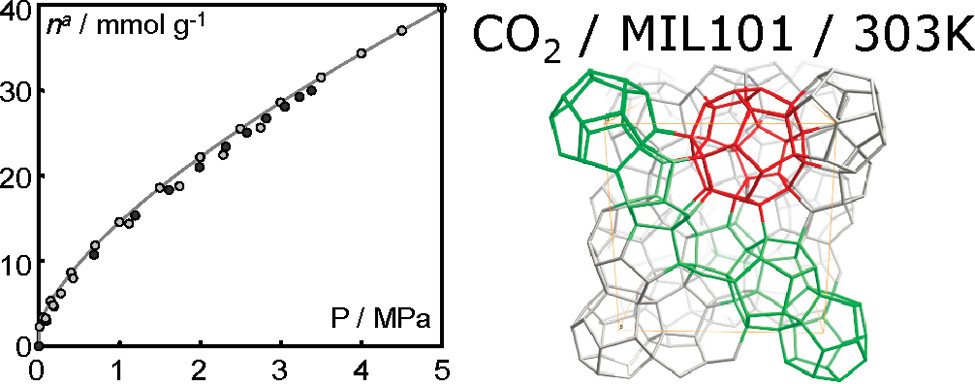
- CO₂ uptake: 28 mmol/g (MIL-101a)、34 mmol/g (MIL-101b)、40 mmol/g (390 cm³/cm³, record) at 5 MPa (MIL-101c).
- Regeneration: Both MOFs were reversible down to 1 bar; complete regeneration was achieved with short secondary vacuum (0.015 Pa) at 303 K, no carbonate formation, mild conditions.
4. Mechanism
- Gas-solid interaction: CO₂ adsorbs first on Cr³⁺ coordinatively unsaturated sites (Lewis acid sites) via strong coordination, then fills residual pores (weak interactions); CH₄ has moderate interactions with MOF surfaces (metal centers and organic linkers) due to no permanent dipole moment.
- MIL-101 activation effect: Unreacted terephthalic acid in MIL-101a blocks active sites; hot ethanol (MIL-101b) and F⁻ treatment (MIL-101c) remove impurities, increase Lewis acid site concentration (500→700→1000 μmol/g) and BET surface area, enhancing CO₂ uptake.
- Henry’s constant: MIL-100 had higher CO₂ Henry’s constant (281 mmol·g⁻¹·mbar⁻¹) than MIL-101c (18 mmol·g⁻¹·mbar⁻¹), indicating stronger CO₂-MIL-100 interaction.
Background:
1. To address the problem of high regeneration cost of traditional adsorbents (zeolites and activated carbons) for separating/storing greenhouse gases (CO₂ and CH₄), previous researchers developed porous MOFs with adjustable pores and mild regeneration conditions, yet their adsorption capacities and interaction strengths with target gases still had room for improvement.
2. The authors in this study proposed optimizing the synthesis and activation methods of MIL-100(Cr) and MIL-101(Cr), and obtained MIL-101c with a record CO₂ uptake capacity and confirmed the good regeneration performance of both MOFs.
Research Content:
1. Synthesis
- MIL-100(Cr): The authors synthesized it using a hydrothermal method: 0.5 g of chromium(VI) oxide, 1.05 g of trimesic acid, 1.0 mL of 5 M hydrofluoric acid solution, and 24 mL of deionized water were mixed, stirred at room temperature, then reacted in a Teflon-lined hydrothermal bomb at 493 K for 4 days; the product was washed and dried, with no additional activation needed.
- MIL-101(Cr): Three samples were prepared:
- MIL-101a (as-synthesized): Hydrothermally synthesized at 493 K for 8 h, followed by 3 h cooling to room temperature.
- MIL-101b: MIL-101a was activated with hot ethanol to remove unreacted terephthalic acid.
- MIL-101c: MIL-101b was treated in 150 mL of 30 mM F⁻ aqueous solution at 333 K for 10 h, then filtered and washed with hot water.
2. Characterizations
1. BET and pore size distribution:
MIL-100: BET surface area 1900 m²/g, pore volume 1.10 cm³/g (at p/p⁰=0.8), free internal diameters of cages ~2.5 nm and 2.9 nm, window sizes 0.55 nm and 0.86 nm, cubic cell volume ~380000 ų.
MIL-101a: BET surface area 2800 m²/g, pore volume 1.37 cm³/g.
MIL-101b: BET surface area 3780 m²/g, pore volume 1.74 cm³/g.
MIL-101c: BET surface area 4230 m²/g, pore volume 2.15 cm³/g, free internal diameters of cages ~2.9 nm and 3.4 nm, window sizes 1.2 nm and 1.6 nm, cubic cell volume ~702000 ų.
2. SEM/TEM tests: The article did not mention SEM/TEM tests for particle size analysis.
3. Other tests:
Microcalorimetry: Enthalpy of CH₄ adsorption at zero coverage ~19 kJ/mol (MIL-100) and ~18 kJ/mol (MIL-101c); CO₂ adsorption enthalpy at zero coverage ~62 kJ/mol (MIL-100) and ~44 kJ/mol (MIL-101c).
FTIR: CO adsorption at 100 K showed MIL-100 had three ν(CO) bands (2207, 2200, 2193 cm⁻¹) for Lewis acid sites, MIL-101 had one ν(CO) band (2196 cm⁻¹); CO₂ adsorption showed ν₃ band (2348 cm⁻¹ for MIL-100, 2351 cm⁻¹ for MIL-101) and no carbonate species.
Manometry and gravimetry: Adsorption isotherms at 303 K confirmed reproducible gas uptake data across three labs.
3. Application
The materials were tested for CO₂ and CH₄ adsorption at 303 K:
- MIL-100: CH₄ uptake ~9.5 mmol/g at 3.5 MPa; CO₂ uptake 18 mmol/g (280 cm³/cm³) at 5 MPa.
- MIL-101:
- CH₄ uptake ~15 mmol/g at 6 MPa (135 cm³/cm³) for all samples.
- CO₂ uptake: 28 mmol/g (MIL-101a)、34 mmol/g (MIL-101b)、40 mmol/g (390 cm³/cm³, record) at 5 MPa (MIL-101c).
- Regeneration: Both MOFs were reversible down to 1 bar; complete regeneration was achieved with short secondary vacuum (0.015 Pa) at 303 K, no carbonate formation, mild conditions.
4. Mechanism
- Gas-solid interaction: CO₂ adsorbs first on Cr³⁺ coordinatively unsaturated sites (Lewis acid sites) via strong coordination, then fills residual pores (weak interactions); CH₄ has moderate interactions with MOF surfaces (metal centers and organic linkers) due to no permanent dipole moment.
- MIL-101 activation effect: Unreacted terephthalic acid in MIL-101a blocks active sites; hot ethanol (MIL-101b) and F⁻ treatment (MIL-101c) remove impurities, increase Lewis acid site concentration (500→700→1000 μmol/g) and BET surface area, enhancing CO₂ uptake.
- Henry’s constant: MIL-100 had higher CO₂ Henry’s constant (281 mmol·g⁻¹·mbar⁻¹) than MIL-101c (18 mmol·g⁻¹·mbar⁻¹), indicating stronger CO₂-MIL-100 interaction.
 Outlook:
This research confirms MIL-100(Cr) and MIL-101(Cr) as excellent candidates for high-pressure greenhouse gas adsorption/separation. MIL-101c’s record CO₂ uptake (40 mmol/g at 5 MPa) and both MOFs’ mild regeneration conditions (energy-saving) overcome traditional adsorbent limitations. Their stable cycling performance and water resistance (unlike zinc-based MOFs) enhance practical application potential in pressure swing adsorption (PSA) for CO₂ capture, supporting energy development and environmental protection goals. Future work may focus on further optimizing MIL-101 activation to remove residual organics and exploring coadsorption behavior of mixed gases.
High Uptakes of CO₂ and CH₄ in Mesoporous Metal-Organic Frameworks MIL-100 and MIL-101
Authors: Philip L. Llewellyn, Sandrine Bourrelly, Christian Serre, Alexandre Vimont, Marco Daturi, Lomig Hamon, Guy De Weireld, Jong-San Chang, Do-Young Hong, Young Kyu Hwang, Sung Hwa Jhung, Gérard Férey
DOI: 10.1021/la800227x
Link: https://pubs.acs.org/doi/10.1021/la800227x
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.
Outlook:
This research confirms MIL-100(Cr) and MIL-101(Cr) as excellent candidates for high-pressure greenhouse gas adsorption/separation. MIL-101c’s record CO₂ uptake (40 mmol/g at 5 MPa) and both MOFs’ mild regeneration conditions (energy-saving) overcome traditional adsorbent limitations. Their stable cycling performance and water resistance (unlike zinc-based MOFs) enhance practical application potential in pressure swing adsorption (PSA) for CO₂ capture, supporting energy development and environmental protection goals. Future work may focus on further optimizing MIL-101 activation to remove residual organics and exploring coadsorption behavior of mixed gases.
High Uptakes of CO₂ and CH₄ in Mesoporous Metal-Organic Frameworks MIL-100 and MIL-101
Authors: Philip L. Llewellyn, Sandrine Bourrelly, Christian Serre, Alexandre Vimont, Marco Daturi, Lomig Hamon, Guy De Weireld, Jong-San Chang, Do-Young Hong, Young Kyu Hwang, Sung Hwa Jhung, Gérard Férey
DOI: 10.1021/la800227x
Link: https://pubs.acs.org/doi/10.1021/la800227x
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

