Product: ZIF-8
Synonyms: MAF-4 ; 2-Methylimidazole zinc salt ; BasoLite Z1200
CAS:59061-53-9
Basic Information
| Unit MF. | C8H10N4Zn | Unit MW. | 227.5818 | ||
| Coordination Metal | Zn | Linkers | 2-Methylimidazole(CAS:693-98-1) | ||
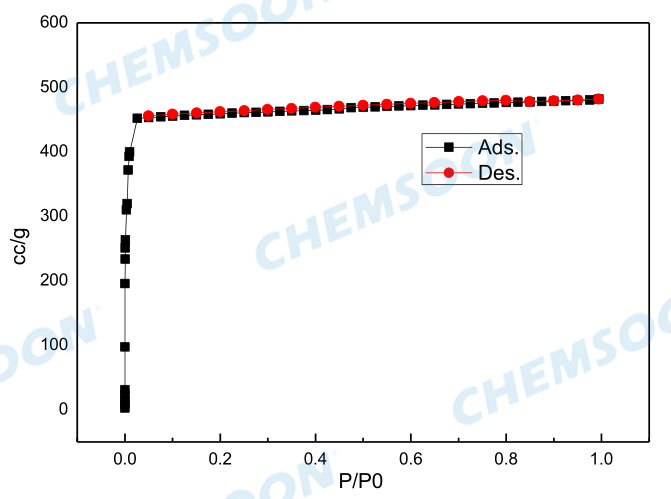
| Aperture | 0.34nm; 1.1nm | Pore volume | 0.66 cm3/g | ||
| Surface Area | BET Specific surface 1500 m2/g, | ||||
| Analog Structure |    |
||||
Product Property
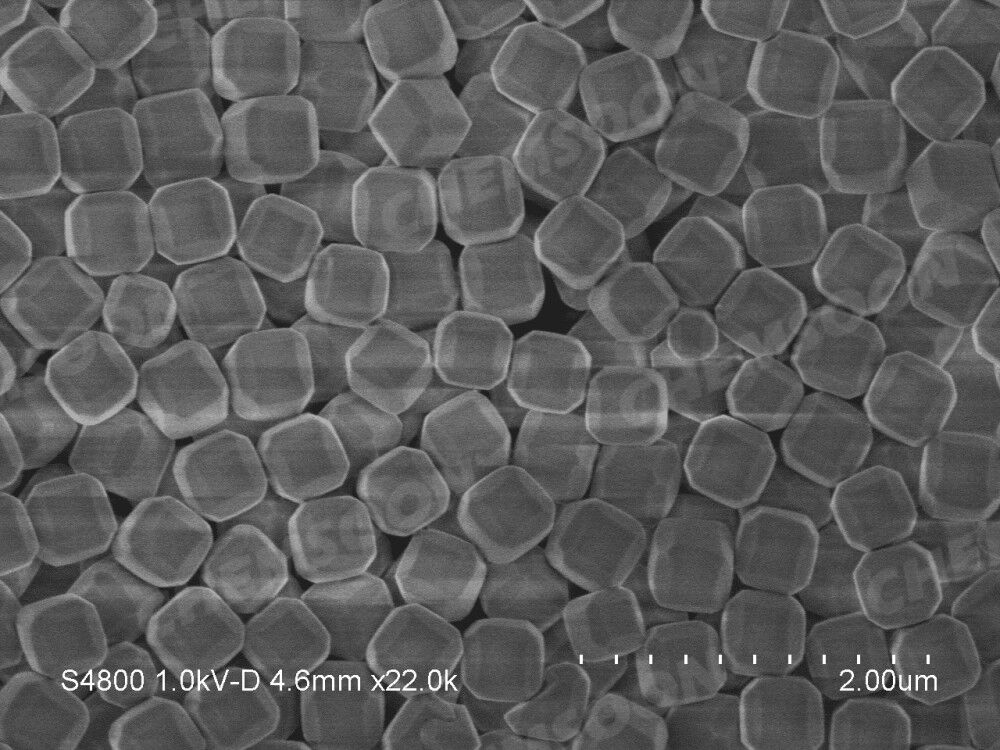
| Appearance | Off white Powder |  |
||
| Particle Size | 300-600nm | |||
Stability
1) ZIF-8 is stable in air, stable in aqueous and strong alkaline conditions, instable in acidic condition
2) High thermal stability, thermal decomposition temperature above 400 ° C
2) High thermal stability, thermal decomposition temperature above 400 ° C
Preservation
1) Keep sealed in dry and cool condition
2) It is recommended to activate for 12 hours at 120 degree in vacuum before gas adsorption test
Other Features
Fluorescence:NA
Applications
1) Gas (such as carbon dioxide) and pollutant adsorption
2) Good drug carrier, which is able to decompose in stomach and release the drug content
2) Good drug carrier, which is able to decompose in stomach and release the drug content
Characterizations
References
1) Huang, Xiao-Chun; Lin, Yan-Yong; Zhang, Jie-Peng; Chen, Xiao-Ming; Angew. Chem. Int. Ed. 2006, 45, 1557-1559, DOI: 10.1002/anie.200503778 ; Ligand-directed strategy for zeolite-type metal-organic frameworks: zinc(II) imidazolates with unusual zeolitic topologies
2) Park, Kyo Sung; Ni, Zheng; Cote, Adrien P.; Choi, Jae Yong; Huang, Rudan; Uribe-Romo, Fernando J.; Chae, Hee K.; O'Keeffe, Michael; Yaghi, Omar M.; Proc. Natl. Acad. Sci. USA 2006, 103, 10186-10191, DOI: 10.1073/pnas.0602439103 ; Exceptional chemical and thermal stability of zeolitic imidazolate frameworks
3) Liu, Chang; Tong, Yu-Long; Yu, Xiao-Qing; Shen, Haixia; Zhu, Zhijie; Li, Qing; Chen, Su; ACS Appl. Mater. Interfaces 2020, 12, 2, 2816–2825, DOI: 10.1021/acsami.9b18012 ; MOF-Based Photonic Crystal Film toward Separation of Organic Dyes;
4) Guang Lu, Shaozhou Li, Zhen Guo, Omar K. Farha, Brad G. Hauser, Xiaoying Qi, Yi Wang, Xin Wang, Sanyang Han, Xiaogang Liu, Joseph S. DuChene, Hua Zhang, Qichun Zhang, Xiaodong Chen, Jan Ma, Say Chye Joachim Loo, Wei D. Wei, Yanhui Yang, Joseph T. Hupp, Fengwei Huo; NATURE CHEMISTRY, 2012, 4; DOI:10.1038/nchem.1272; Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation;
5) Yichang Pan, Yunyang Liu, Gaofeng Zeng, Lan Zhao, Zhiping Lai*;ChemComm, 2011, 47, 2071–2073; DOI:10.1039/c0cc05002d; Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system;
6) Helge Bux, Fangyi Liang, Yanshuo Li, Janosch Cravillon, Michael Wiebcke, Ju¨rgen Caro; J. AM. CHEM. SOC., 2009, 131, 44; DOI:10.1021/ja907359t; Zeolitic Imidazolate Framework Membrane with Molecular Sieving Properties by Microwave-Assisted Solvothermal Synthesis;
7) D. Fairen-Jimenez, S. A. Moggach, M. T. Wharmby, P. A. Wright, S. Parsons, T. Düren; Journal of the American Chemical Society, 2011, 133, 8900–8902; DOI:10.1021/ja202154j; Opening the Gate: Framework Flexibility in ZIF-8 Explored by Experiments and Simulations;
8) Janosch Cravillon, Roman Nayuk, Sergej Springer, Armin Feldhoff, Klaus Huber, Michael Wiebcke; Chemistry of Materials, 2011, 23, 2130-2141; DOI:10.1021/cm103571y; Controlling Zeolitic Imidazolate Framework Nano- and Microcrystal Formation: Insight into Crystal Growth by Time-Resolved In Situ Static Light Scattering;
9) Guang Lu and Joseph T. Hupp*; J. AM. CHEM. SOC., 2010, 132, 23, 7832-7833; DOI:10.1021/ja101415b; Metal-Organic Frameworks as Sensors: A ZIF-8 Based Fabry-Pérot Device as a Selective Sensor for Chemical Vapors and Gases;
10) Kui Shen, Lei Zhang, Xiaodong Chen, Lingmei Liu, Daliang Zhang, Yu Han, Junying Chen, Jilan Long, Rafael Luque, Yingwei Li, Banglin Chen; Science, 2018, 359, 6372; DOI :10.1126/science.aao3403; Ordered macro-microporous metal-organic framework single crystals;