Home >
News > ZIF-8-Based Sensor for Chemicals
ZIF-8-Based Sensor for Chemicals
Summary:
The authors from Department of Chemistry, Northwestern University (Evanston, IL 60208) developed a ZIF-8 thin film with characteristics of controllable thickness, high chemical robustness, thermal stability, and microporous structure (sodalite-type, 11.6 Å cavities, 3.4 Å pore apertures), achieving selective sensing performance for chemical vapors and gases in the application of chemical sensing field.
Background:
1. To address the challenge of signal transduction in metal-organic framework (MOF)-based sensing (MOF cavities are too small to tailor with reporter molecules, leading to few reports of MOF-based sensors), previous researchers mainly used framework luminescence (with signal transduction via luminescence quenching) but lacked alternative effective transduction strategies.
2. The authors in this study proposed an innovative method: configuring ZIF-8 as a transparent thin film and using Fabry-Pérot interference peak shifts (reflecting refractive index changes) for signal readout, successfully developing a selective sensor for chemical vapors and gases.
Research Content:
1. Synthesis
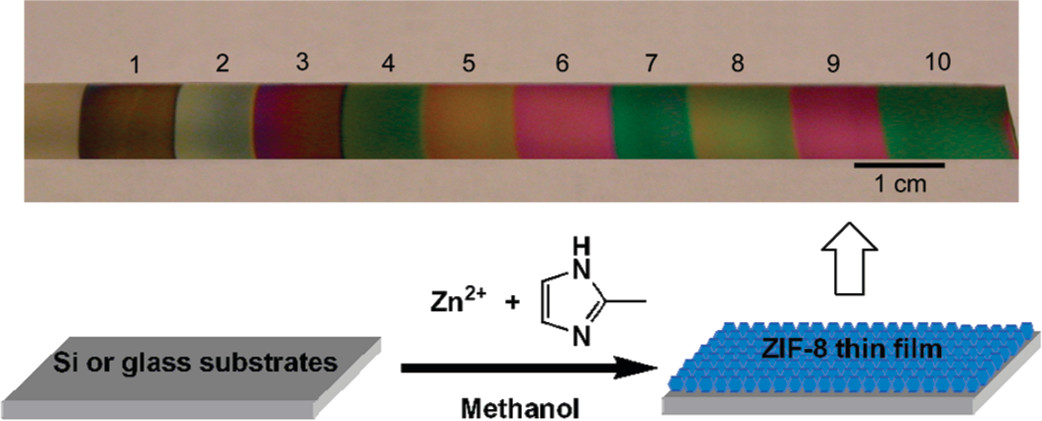
The authors synthesized ZIF-8 thin films using a room-temperature immersion method:
- Clean glass/silicon substrates (cleaned with piranha solution, rinsed with distilled water, dried under nitrogen) were immersed in a fresh methanolic mixture of 25 mM Zn(NO₃)₂·6H₂O and 50 mM 2-methylimidazole (mIm) for 30 minutes at room temperature.
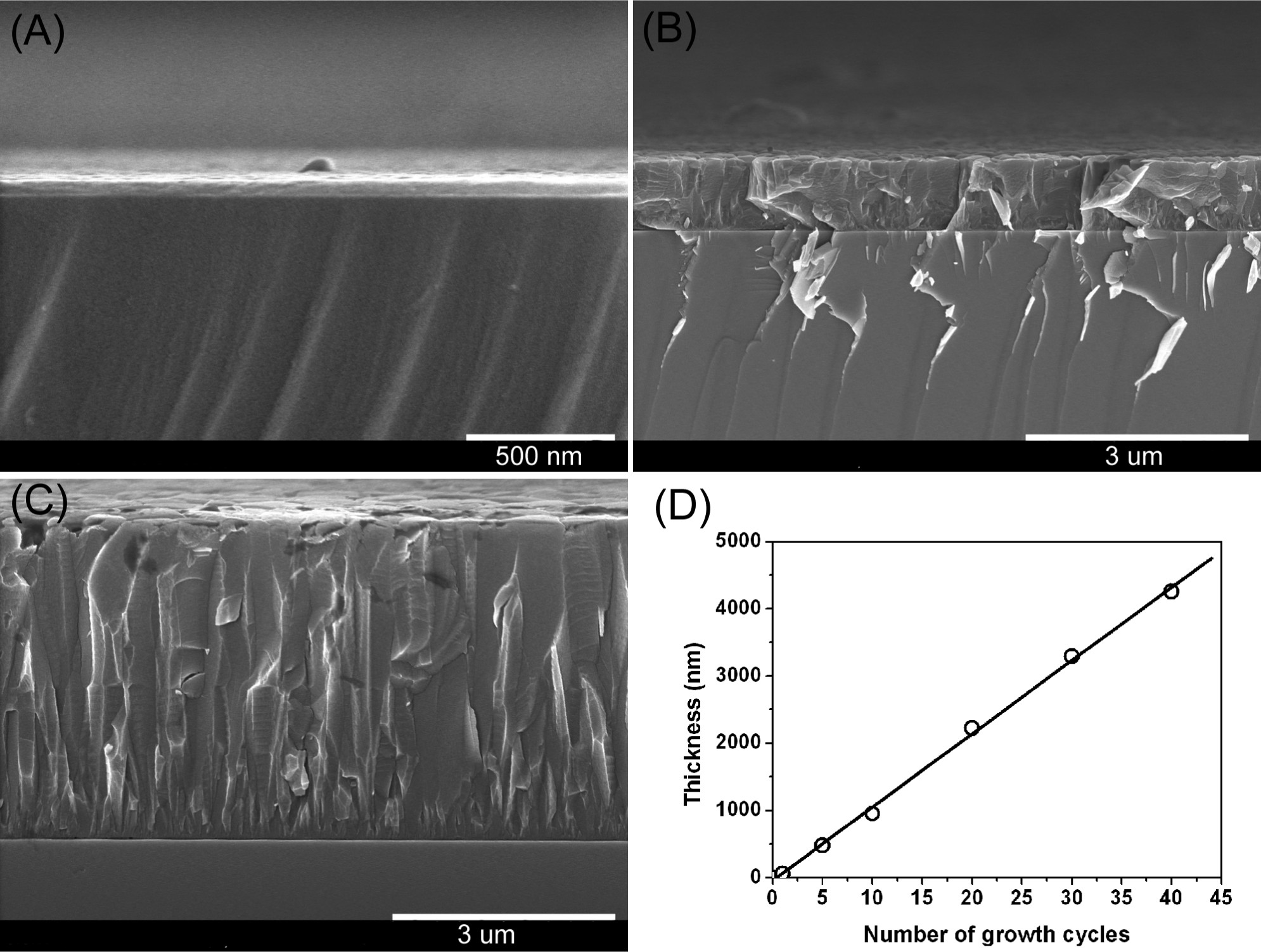
- Thicker films were obtained by repeating the process with fresh solutions (≈50 nm for 1 cycle, ≈100 nm per additional cycle).
2. Characterizations
1) BET and pore size distribution: The ZIF-8 powder sample had a BET surface area (Sᵦₑₜ) of 1530 m²/g and a micropore volume (Vₘᵢcᵣₒ) of 0.59 cm³/g (estimated by SF method); nitrogen physisorption isotherms (77 K) confirmed its microporous nature.
2) SEM tests: Cross-sectional SEM showed film thickness increased by ≈100 nm per growth cycle (1 cycle: ≈50 nm, 10 cycles: ≈1000 nm, 40 cycles: thicker); top-view SEM revealed uniform film morphology (cracks in EDXS mapping due to 15 kV high voltage).
3) Other tests:
- XRD: Out-of-plane XRD confirmed ZIF-8 crystal structure (matches simulation) and crystallinity improved with more cycles; in-plane XRD of 40-cycle film verified orientation; variable-temperature XRD (25–350 °C in air) showed thermal stability.
- FT-IR ATR: 40-cycle film exhibited characteristic peaks (e.g., 1623 cm⁻¹, 1585 cm⁻¹) of ZIF-8, confirming chemical composition.
- TGA: Powder sample showed thermal stability (weight loss consistent with solvent removal, no significant decomposition in relevant temperature range).
- QCM: Gold electrode QCM measurements showed rapid initial film growth, slowing later, and ≈90% reaction completion in 30 minutes.
- UV-vis: Transmission spectra showed Fabry-Pérot interference peaks; refractive index of evacuated ZIF-8 was ≈1.39.
3. Application
The ZIF-8 thin film (10-cycle, ≈1000 nm thick) was tested as a chemical vapor/gas sensor, with results:
- Gas sensing: Exposed to propane (nitrogen as diluent), interference peaks (originally 612 nm) red-shifted up to 49 nm; shift extent depended on propane partial pressure, reversible within 1 minute; volume fraction of propane in framework was ≈0.13 (1 atm pure propane).
- Vapor sensing: Responsive to ethanol vapor (red-shifted peaks) but unresponsive to water vapor (consistent with ZIF-8 hydrophobicity); ethanol/water mixture tests showed concentration-dependent shifts (saturating at ≈40% ethanol volume); volume fraction of ethanol in framework was ≈0.25 (pure ethanol, matching single-crystal XRD void volume fraction ≈0.20).
- Selectivity: Sensed linear n-hexane but not sterically bulky cyclohexane (due to 3.4 Å pore apertures); detection limit for ethanol in water was ≈0.3 vol% (≈100 ppm vapor, 1 nm spectrophotometer resolution).
4. Mechanism
- Sensing mechanism: ZIF-8 is microporous (vacuum in cavities, nᵥₐc=1); polarizable analyte molecules enter cavities, displace vacuum, and increase the film’s effective refractive index (n). From Fabry-Pérot equation (mλ=2nl, m=integer, λ=wavelength, l=film thickness), increased n causes red shifts of interference peaks; peak shifts are quantified to calculate analyte volume fraction.
- Volume fraction calculation: Using effective refractive indices before (nᵥₐc) and after (nₐ) analyte exposure (from UV-vis interference valleys via n=Nλ₁λ₂/[2l(λ₁-λ₂)]), volume fraction Vₐ=(nₐ² - nᵥₐc²)/(nₐ⁰² - nᵥₐc²) (nₐ⁰=analyte refractive index, nᵥₐc=1), which correlates with analyte concentration.
- Selectivity mechanism: Pore aperture size (3.4 Å) excludes sterically larger molecules (e.g., cyclohexane); hydrophobic framework repels water, leading to ethanol/water selectivity.
Outlook:
This research achieves three key contributions: 1) Develops a simple, controllable ZIF-8 thin film synthesis method (room temperature, rapid growth, thickness control, no special substrate modification), solving the problem of submicrometer-thickness control in MOF films. 2) Proposes a novel MOF sensing strategy using Fabry-Pérot interference (refractive index readout), circumventing the need for molecular reporters and expanding MOF sensor signal transduction methods. 3) The ZIF-8 sensor exhibits high selectivity, reversibility, and low detection limit, providing a practical platform for selective chemical vapor/gas sensing, with potential for optimizing sensor performance (e.g., thickness, substrate) and extending to other MOFs for broader analytes.
Metal-Organic Frameworks as Sensors: A ZIF-8 Based Fabry-Pérot Device as a Selective Sensor for Chemical Vapors and Gases
Authors: Guang Lu and Joseph T. Hupp*
DOI: 10.1021/ja101415b
Link: https://pubs.acs.org/doi/10.1021/ja101415b
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

