Home >
News > Controlling ZIF Nano-/Microcrystal Growth
Controlling ZIF Nano-/Microcrystal Growth
Summary:
The authors from Leibniz Universität Hannover (Germany), Universität Paderborn (Germany) developed ZIF-8 nano- and microcrystals with tunable size (≈10–65 nm for nanocrystals, ≈1 μm for microcrystals), good thermal stability, large surface area, and dual micro-mesoporosity (for nanocrystals), achieving size-controlled synthesis and insight into crystal growth mechanisms in the field of metal-organic frameworks (MOFs).
Background:
1. To address the lack of rational size- and shape-controlled synthesis of zeolitic imidazolate framework (ZIF) materials and insufficient understanding of their crystallization mechanisms, previous researchers prepared ZIF nanocrystals via empirical methods (e.g., adding polymers) but failed to clarify growth mechanisms; carboxylate-based MOF synthesis used coordination modulation but only with same-functional ligands, not extending to ZIFs.
2. The authors proposed an innovative method: using excess bridging bidentate ligand (2-methylimidazole, Hmim) and auxiliary monodentate modulating ligands (sodium formate, 1-methylimidazole, n-butylamine) with different functionalities, realizing rapid room-temperature synthesis of ZIF-8 with tunable size (≈10 nm–1 μm) and revealing crystallization mechanisms via in-situ static light scattering (SLS).
Research Content:
1. Synthesis
-ZIF-8 nanocrystals (no modulating ligand): Dissolve Zn(NO₃)₂·6H₂O (2.469 mmol) and Hmim (9.874 mmol) in 50 mL methanol (MeOH) each, mix under stirring, centrifuge after 24 h, wash with MeOH, and dry (yields ≈65 nm rhombic dodecahedral nanocrystals).
-ZIF-8 microcrystals (sodium formate/1-methylimidazole as ligand): Dissolve Zn(NO₃)₂·6H₂O (2.469 mmol) in 50 mL MeOH; dissolve Hmim (9.874 mmol) and sodium formate/1-methylimidazole (9.874 mmol) in 50 mL MeOH, mix, filter after 24 h, wash, and dry (yields ≈1 μm rhombic dodecahedral microcrystals).
-ZIF-8 nanocrystals (n-butylamine as ligand): Similar to above, replace sodium formate/1-methylimidazole with n-butylamine (9.874 mmol), centrifuge after 24 h (yields ≈18 nm nearly spherical nanocrystals); adjust molar ratios (Zn/Hmim/n-butylamine/MeOH) to tune size to 9–65 nm.
2. Characterizations
1.BET and pore size distribution: 18 nm ZIF-8 nanocrystals have BET surface area ≈1617 m²/g (comparable to macrocrystals); mesopore size distribution centers at ≈8 nm, showing dual micro-mesoporosity.
2.SEM/TEM tests:
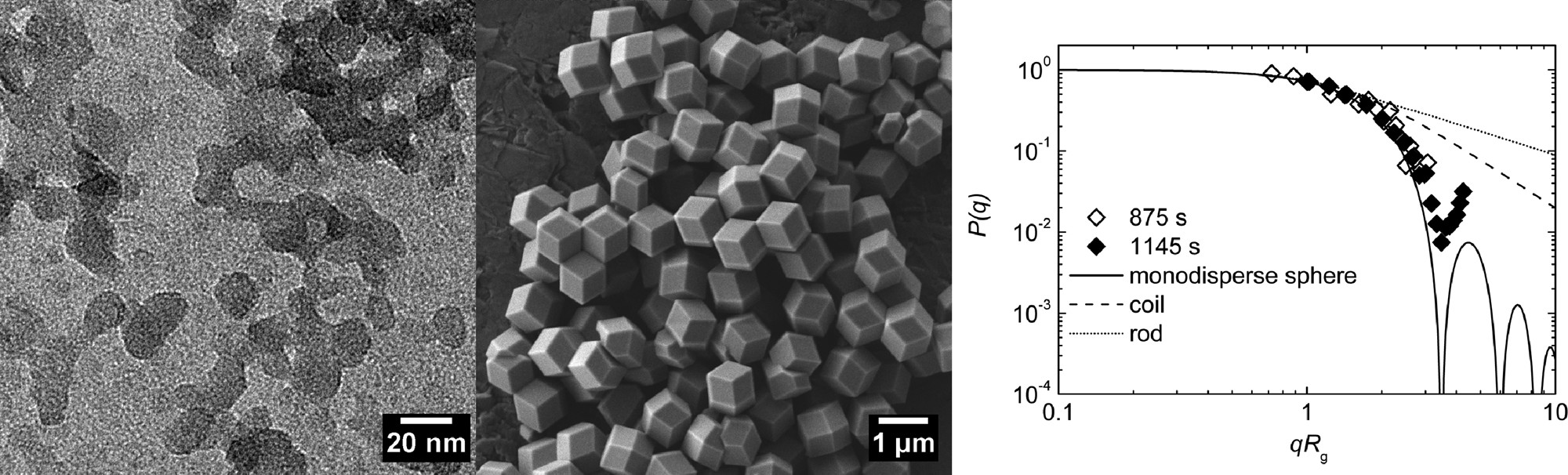
- No ligand: ≈65 nm (±13 nm) rhombic dodecahedra, narrow size distribution after 1 h.
- Sodium formate/1-methylimidazole: ≈1 μm rhombic dodecahedra.
- n-butylamine: ≈18 nm (±4 nm) nearly spherical particles, with small secondary aggregates (max size ≈88 nm).
3.Other tests:
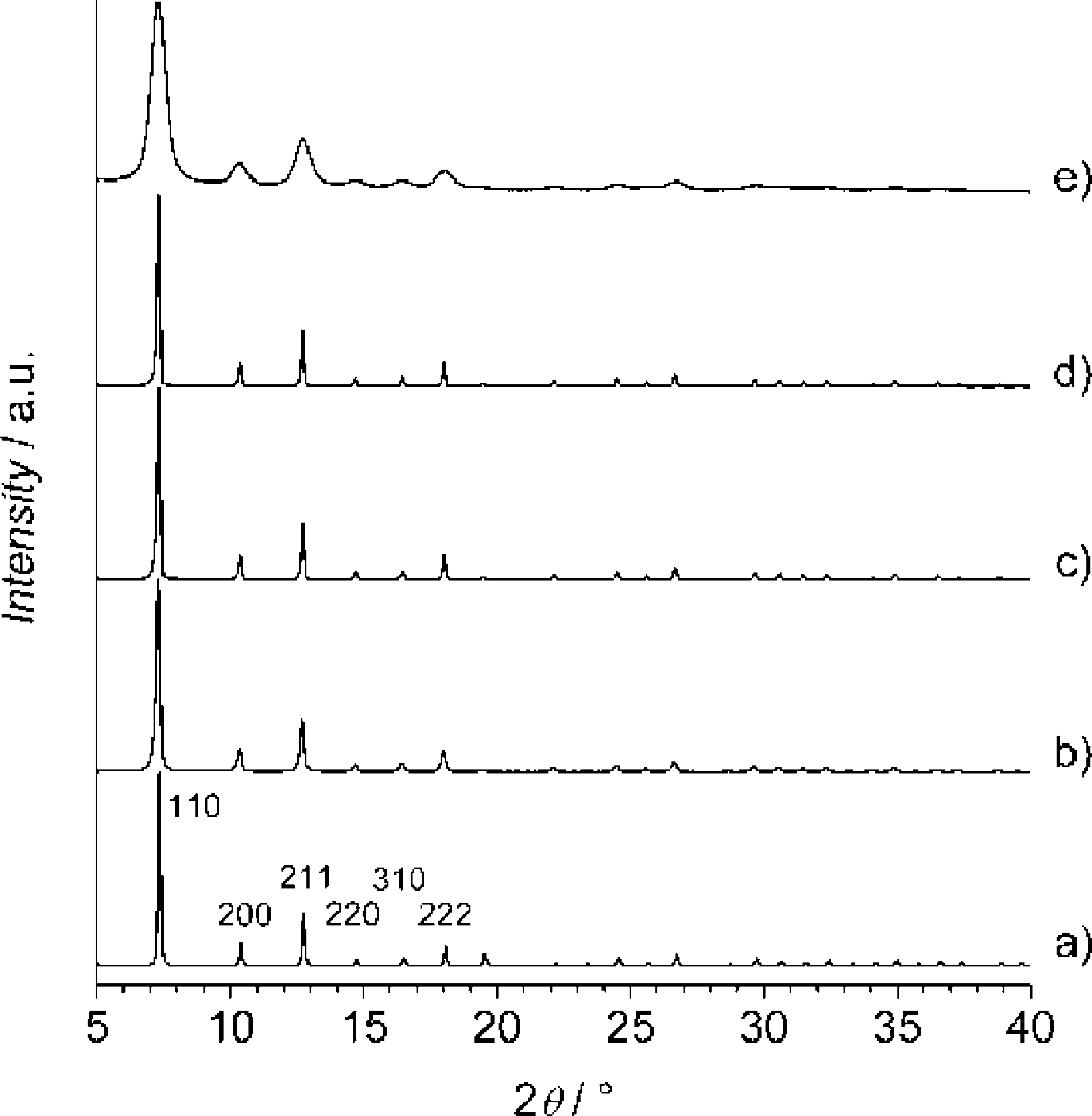
- XRD: All products are pure-phase ZIF-8; variable-temperature XRD shows stability up to 250°C (18 nm nanocrystals) or 300°C (larger crystals/microcrystals).
- TG/DTA: Total mass loss ≈65% (matches calculated 64%), decomposing to hexagonal ZnO; no solvent loss before decomposition (easy activation).
- SAXS: 65 nm nanocrystals (polydisperse spheres, radius 44 nm, polydispersity 0.28); 18 nm nanocrystals (primary size 18 nm, Rg=27 nm).
- FT-IR: Confirms ZIF-8 structure (characteristic peaks of imidazole ligands).
3. Application
Not explicitly tested in specific applications, but the materials have potential for gas separation, adsorption, catalysis, and membrane fabrication (inferred from ZIF-8’s typical properties and related research references).
4. Mechanism
-Nanocrystal growth: Continuous slow nucleation + fast growth (seconds scale); size distribution "narrows" (focuses) to narrow range at intermediate stages (1 h) due to colloidal stabilization by excess Hmim, then "broadens" (defocuses) via Ostwald ripening.
-Microcrystal growth: Two-stage mechanism—early coalescence (α=0.29, Rg ∝ Mw^α) and later monomer attachment (α=0.15–0.19); shape evolves from cubes ({100} faces) to rhombic dodecahedra ({110} faces) in later stages.
-Modulating ligand function: Ligands with pKa < 10.3 (sodium formate, 1-methylimidazole) compete with Hmim, reducing nucleation rate (larger crystals); pKa > 10.3 (n-butylamine) accelerates deprotonation, increasing nucleation rate (smaller crystals).
Outlook:
This research realizes rational size control of ZIF-8 (10 nm–1 μm) via a novel modulation strategy, clarifies crystallization mechanisms (nucleation/growth/coalescence) using in-situ SLS, and provides a basis for controlled synthesis of ZIF bulk materials, membranes, and films. The dual-porosity nanocrystals and easy activation also expand their application potential in adsorption and catalysis.
Controlling Zeolitic Imidazolate Framework Nano- and Microcrystal Formation: Insight into Crystal Growth by Time-Resolved In Situ Static Light Scattering
Authors: Janosch Cravillon, Roman Nayuk, Sergej Springer, Armin Feldhoff, Klaus Huber, Michael Wiebcke
DOI: 10.1021/cm103571y
Link: https://pubs.acs.org/doi/10.1021/cm103571y
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

