Home >
News > Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation
Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation
Summary:
The authors from Nanyang Technological University, Northwestern University, National University of Singapore, University of Florida, and Institute of Materials Research and Engineering developednanoparticle/zeolitic imidazolate framework-8 (ZIF-8) composites with characteristics of well-dispersed nanoparticles, full confinement, and preserved MOF porosity, achieving results in catalytic, magnetic, and optical applications.
Background:
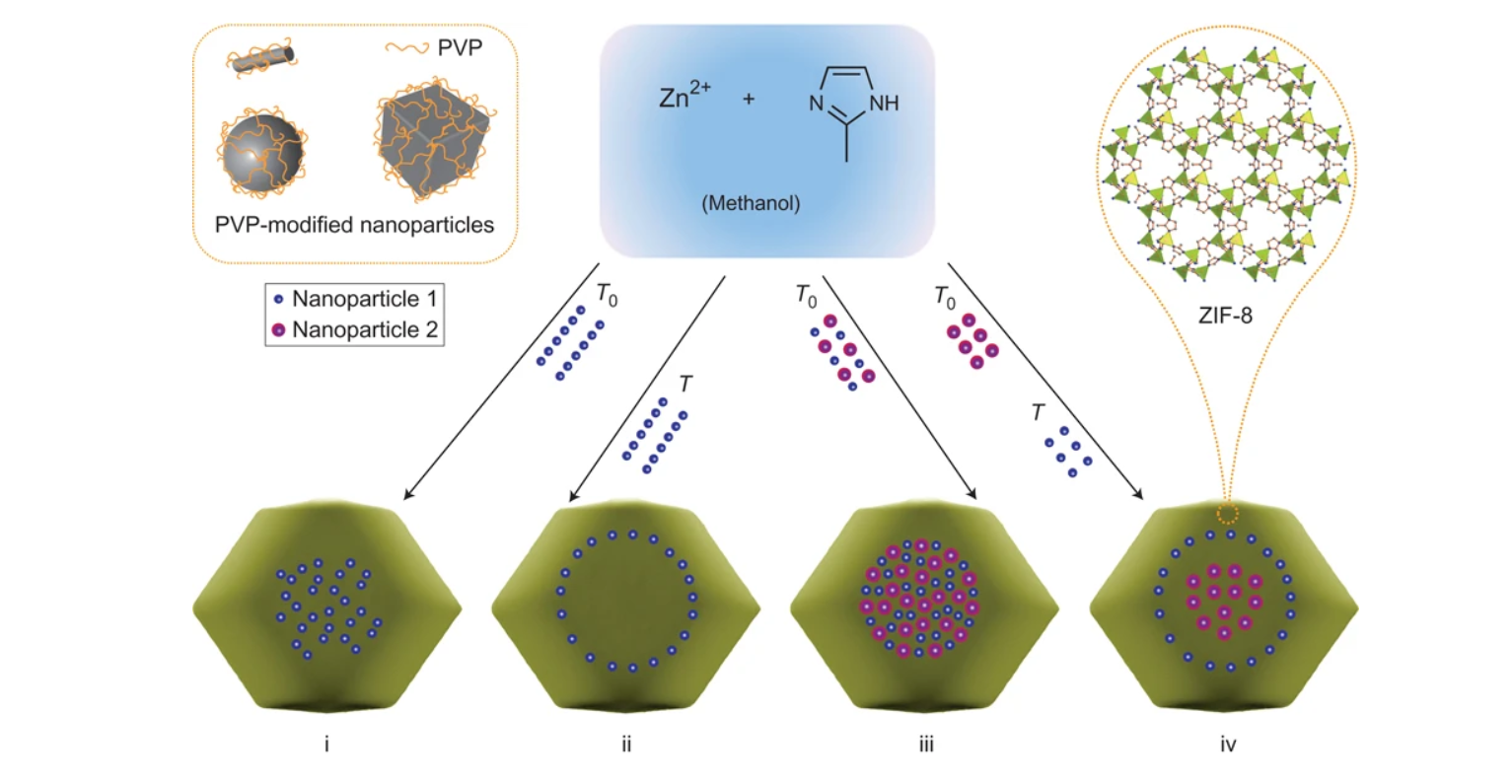
1. To address the challenge of controlling the size, composition, dispersion, spatial distribution, and confinement of nanoparticles in metal–organic frameworks (MOFs), previous researchers prepared nanoparticle/MOF composites via two methods: using MOFs as templates to generate nanoparticles in cavities, and encapsulating presynthesized nanoparticles. However, these methods failed to fully confine nanoparticles, control their spatial distribution, or avoid agglomeration.
2. The authors proposed an innovative strategy—surface modification of nanoparticles with polyvinylpyrrolidone (PVP) and optimization of ZIF-8 crystallization—to encapsulate various nanoparticles in ZIF-8, achieving well-dispersed, fully confined nanoparticles and controllable spatial distribution.
Research Content:
1. Synthesis
-Nanoparticle modification: Synthesized Au (13 nm, 34 nm), Pt (2.5 nm, 3.3 nm, 4.1 nm), Fe₃O₄ (8 nm), CdTe (2.8 nm), NaYF₄ (24 nm, 50×310 nm rods), Ag cubes (160 nm), and polystyrene (PS) spheres (180 nm) via established methods, then modified their surfaces with PVP (during or after synthesis) to enhance stability and affinity for ZIF-8.
-Composite preparation: Mixed PVP-modified nanoparticle solutions with methanolic zinc nitrate (25 mM) and 2-methylimidazole (25 mM) at room temperature, reacted for 24 hours without stirring, centrifuged, washed with methanol, and vacuum-dried to obtain nanoparticle/ZIF-8 composites.
2. Characterizations
1.BET and pore size distribution: Pure ZIF-8 had a BET surface area of 1643 m²/g; composites (e.g., 3.5% 2.5 nm Pt/ZIF-8: 1501 m²/g, 0.7% 3.3 nm Pt/ZIF-8: 1568 m²/g) showed slightly reduced BET areas but retained ZIF-8’s microporosity (type I N₂ isotherms) and pore-size distribution (no alteration from ZIF-8’s 11.6 Å cavities and 3.4 Å apertures).
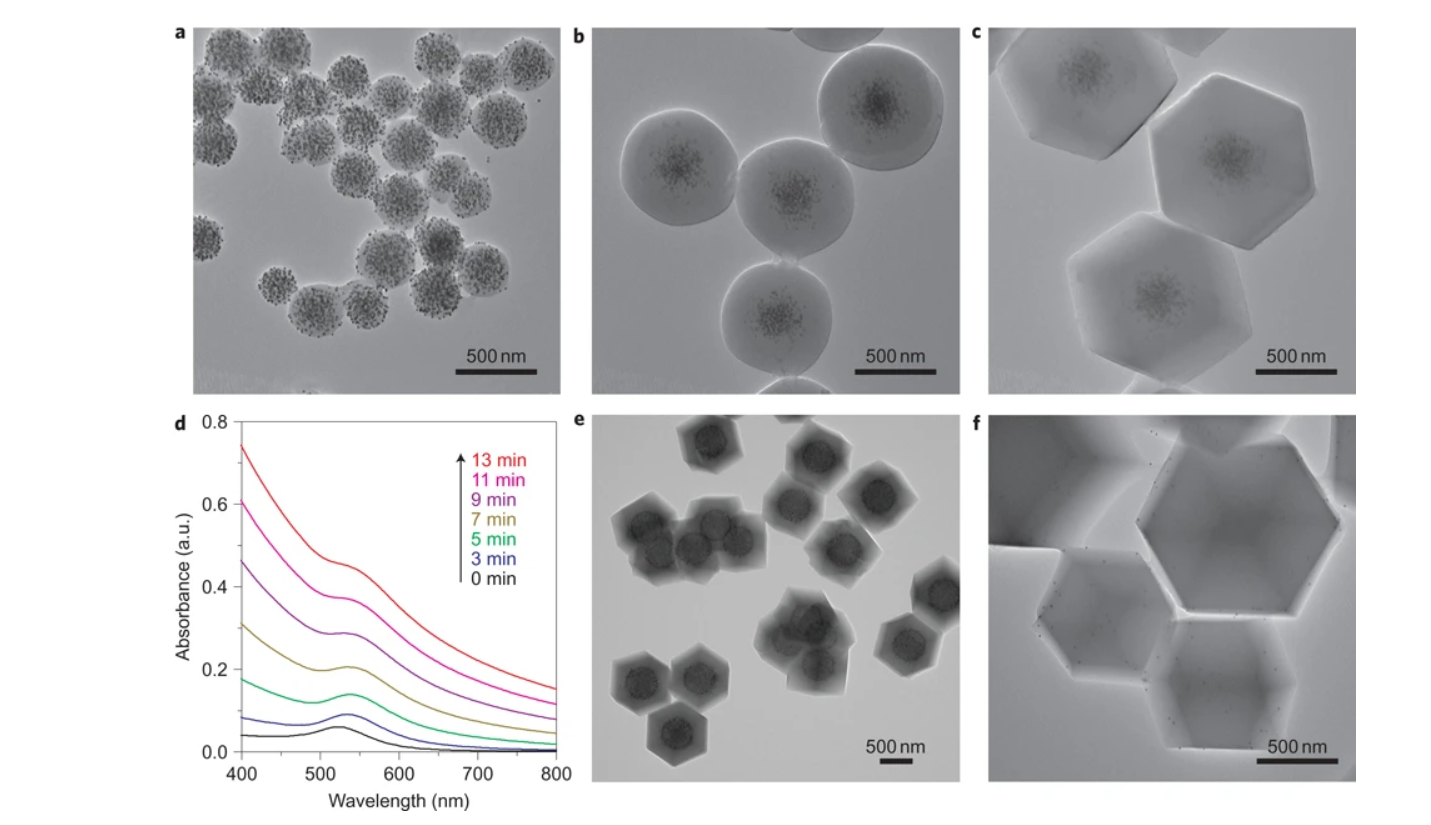
2.SEM/TEM tests: SEM showed composite crystals were ~1.1 μm rhombic dodecahedra (74%) and intergrown crystals (26%); TEM revealed nanoparticles (e.g., 13 nm Au, 3.3 nm Pt) were well-dispersed and fully encapsulated in ZIF-8, with no unencapsulated particles.
3.Other tests:
- XRD: All composites exhibited ZIF-8’s diffraction pattern (no clear nanoparticle peaks due to low concentration/small size).
- TGA: Composites (e.g., 3.5% 2.5 nm Pt/ZIF-8) were less thermally stable than pure ZIF-8 (17% weight loss at 500°C vs. 2.4% for ZIF-8) due to PVP decomposition (Tₐ=435°C).
- Magnetic measurement: Fe₃O₄/ZIF-8 showed superparamagnetism (no hysteresis) and could be collected via magnetic field.
- Photoluminescence: NaYF₄/ZIF-8 emitted green light via near-infrared upconversion; CdTe/ZIF-8 emitted green light (529 nm, slightly shifted from free CdTe’s 532 nm).
3. Application
-Catalysis:
- CO oxidation: 3.3 nm Pt/ZIF-8 started catalysis at 130°C, achieving ~100% conversion at 200°C.
- Alkene hydrogenation: Pt/ZIF-8 catalyzed n-hexene hydrogenation (7.3%–9.6% conversion over 3 runs) but not cis-cyclooctene/trans-2-hexene (size/regioselectivity from ZIF-8’s 3.4 Å apertures).
-Magnetism: Fe₃O₄/ZIF-8 could be rapidly separated from solvent suspensions via magnetic field.
-Optics: CdSe/ZIF-8 showed molecular sieving—2-mercaptoethanol quenched its luminescence, but bulky cyclohexanethiol did not.
4. Mechanism
-Encapsulation mechanism: PVP on nanoparticles (polar pyrrolidone rings, apolar groups) enabled adsorption on growing ZIF-8 surfaces via weak coordination (C=O with Zn in ZIF nodes) and hydrophobic interactions (apolar groups with ZIF ligands), avoiding heterogeneous nucleation. Excess free PVP inhibited adsorption (competitive binding), confirming PVP’s role.
-Performance mechanism: ZIF-8’s microporosity provided molecular sieving (size/regioselectivity in catalysis/luminescence), while encapsulated nanoparticles retained their intrinsic properties (catalytic activity of Pt, magnetism of Fe₃O₄, luminescence of NaYF₄/CdTe).
Outlook:
This research innovatively solves key challenges in nanoparticle/MOF composite preparation, provides a universal method for encapsulating diverse nanoparticles in ZIF-8, and expands MOF-based materials’ applications in catalysis, magnetic separation, and optical sensing, laying a foundation for functional hybrid materials.
Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation
Authors: Guang Lu, Shaozhou Li, Zhen Guo, Omar K. Farha, Brad G. Hauser, Xiaoying Qi, Yi Wang, Xin Wang, Sanyang Han, Xiaogang Liu, Joseph S. DuChene, Hua Zhang, Qichun Zhang, Xiaodong Chen, Jan Ma, Say Chye Joachim Loo, Wei D. Wei, Yanhui Yang, Joseph T. Hupp, Fengwei Huo
DOI: 10.1038/nchem.1272
Link: https://www.nature.com/naturechemistry/articles/nchem.1272
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

