Home >
News > Ordered macro-microporous metal-organic framework single crystals
Ordered macro-microporous metal-organic framework single crystals
Summary:
The authors fromSouth China University of Technology (China),King Abdullah University of Science and Technology (Saudi Arabia),University of Córdoba (Spain), andUniversity of Texas at San Antonio (USA) developed ordered macro-microporous metal-organic framework (MOF) single crystals (taking SOM-ZIF-8 as a proof of concept), achieving superior catalytic activity, recyclability, and mass diffusion properties in the application of bulky-molecule reactions (e.g., Knoevenagel reaction).
Background:
1. To address the limitation that most reported MOFs only have micropores (restricting applications in diffusion-limited processes) and the lack of methods to prepare MOF single crystals with both ordered macropores (diameter >50 nm) and single crystallinity, previous researchers used strategies like templating, etching, and extendable ligands. These methods either failed to form ordered macro-microporous MOF single crystals, caused mesopore collapse after guest removal, or only produced intergrown MOF polycrystals with template-duplicated macropores.
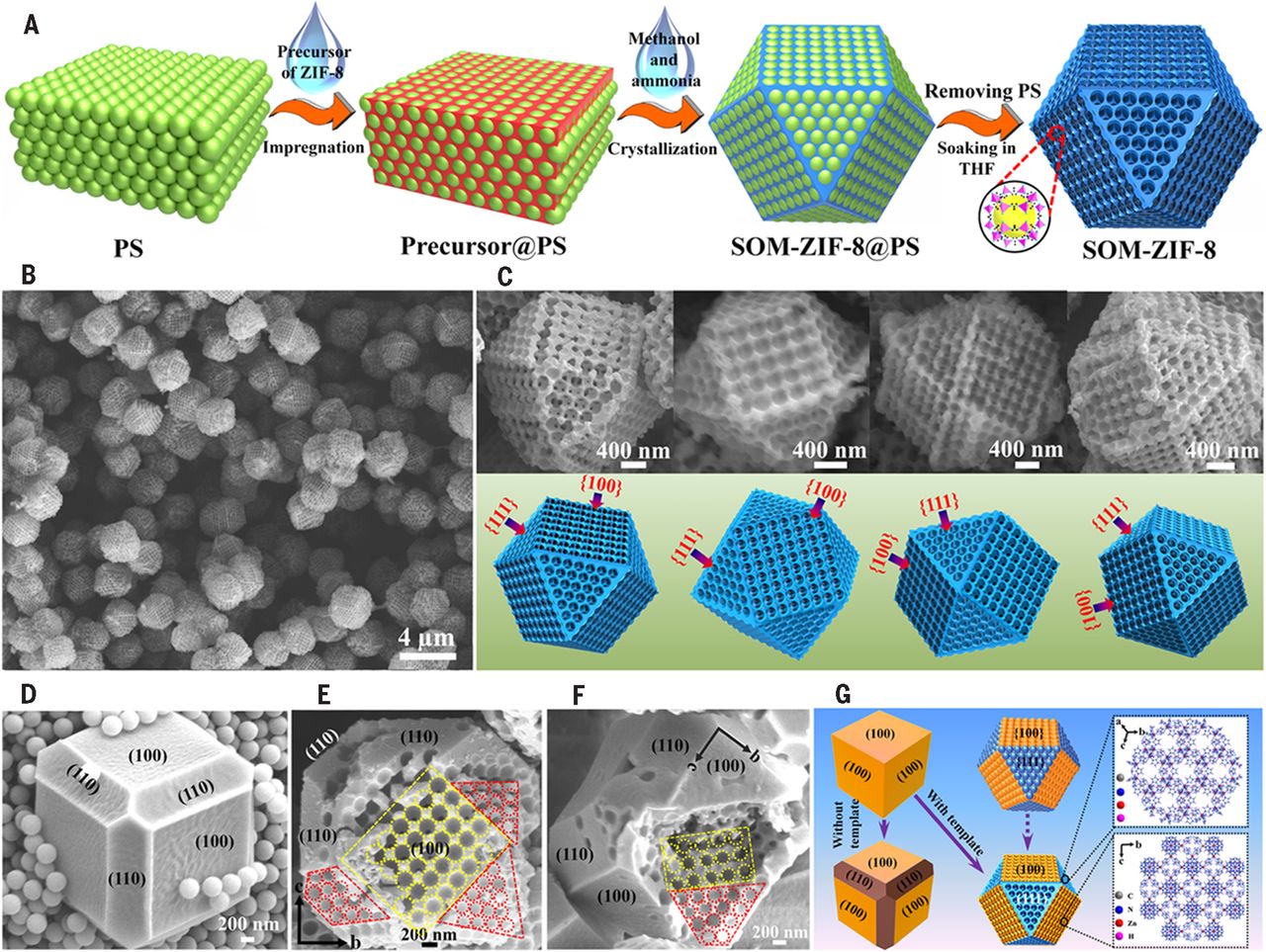
2. The authors proposed an innovative methodology combining a polystyrene (PS) nanosphere monolith template and a double-solvent (methanol/ammonia water)-induced heterogeneous nucleation approach, successfully synthesizing 3D-ordered macro-microporous MOF single crystals (SOM-ZIF-8) with robust structures.
Research Content:
1. Synthesis
The authors synthesized SOM-ZIF-8 through the following steps:
1. Assemble monodisperse PS spheres into highly ordered 3D PS monoliths.
2. Fill ZIF-8 precursors (2-methylimidazole and Zn(NO₃)₂) into the PS monolith interstices to form "precursor@PS" monoliths.
3. Soak the "precursor@PS" monoliths in a CH₃OH/NH₃·H₂O mixed solvent (NH₃·H₂O induces rapid crystallization, while CH₃OH stabilizes precursors and balances nucleation/growth) to realize in-situ growth of oriented 3D-ordered ZIF-8 single crystals in PS template voids.
4. Remove the PS template (via soaking in THF) to obtain SOM-ZIF-8, where the feed molar ratio of 2-methylimidazole/Zn(NO₃)₂ (x) and NH₃·H₂O volume fraction (y) define its notation (e.g., SOM-ZIF-8(3, 0.5)).
2. Characterizations
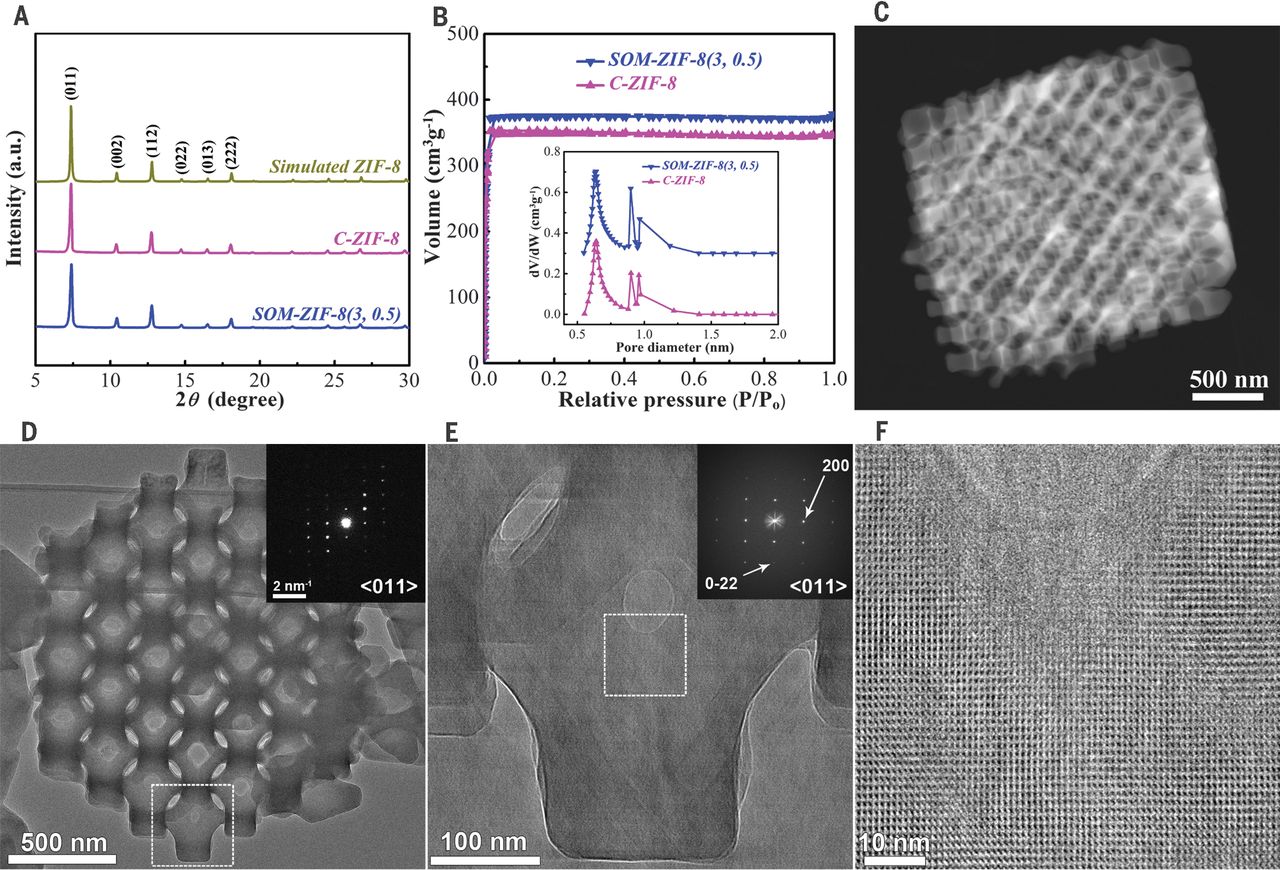
1.BET and pore size distribution:
- SOM-ZIF-8(3, 0.5) has a Brunauer-Emmett-Teller (BET) surface area of 1540 m²/g and a micropore volume of 0.59 cm³/g, higher than conventional ZIF-8 (C-ZIF-8: 1397 m²/g, 0.55 cm³/g).
- It exhibits a type I nitrogen adsorption isotherm (confirming microporous structure) and a regular macroporous distribution (average throat size ~80 nm via mercury intrusion porosimetry; average interconnected macropore diameter ~270 nm, matching PS latex size).
2.SEM/TEM tests:
- SEM shows SOM-ZIF-8(3, 0.5) has a tetrakaidecahedron morphology (inherited {100} and {111} planes of the PS template) with oriented 3D-ordered macropores; particle size can be tuned from ~1.3 μm to ~4.3 μm by adjusting NH₃·H₂O volume fraction (0.33 to 0.67).
- HAADF-STEM confirms ordered PS templates are imprinted into SOM-ZIF-8 crystals; selected-area electron diffraction (SAED) and high-resolution TEM (HRTEM) reveal its single-crystalline nature (uniform lattice fringes, no domain boundaries, consistent FT patterns).
3.Other tests:
- X-ray diffraction (XRD) shows SOM-ZIF-8(3, 0.5) only has ZIF-8 characteristic reflections, confirming phase purity and good crystallinity.
- Elemental mapping demonstrates homogeneous distribution of C, N, and Zn in SOM-ZIF-8 crystals.
3. Application
The material was tested in the Knoevenagel reaction (benzaldehydes + malononitriles) and compared with C-ZIF-8, polycrystal hollow ZIF-8 (PH-ZIF-8), and disordered macroporous ZIF-8 (M-ZIF-8):
1. Catalytic activity: SOM-ZIF-8(3, 0.33) completes benzaldehyde conversion in 2 hours, outperforming C-ZIF-8 (8 hours), PH-ZIF-8, M-ZIF-8, and state-of-the-art heterogeneous catalysts (e.g., amino-functionalized molecular sieves, UiO-66-NH₂).
2. Recyclability: SOM-ZIF-8(3, 0.33) can be reused ≥7 times, with benzaldehyde conversion only decreasing from 94.6% to 87.0%; PH-ZIF-8 loses ≥5% activity per cycle due to structural collapse.
3. Substrate adaptability: SOM-ZIF-8(3, 0.33) shows higher activity than C-ZIF-8 for all tested substituted benzaldehydes, especially those with bulky groups.
4. Mechanism
1.Synthesis mechanism: The PS monolith template guides oriented crystal growth (forming tetrakaidecahedron morphology and matching crystal faces with macropore arrangements), while the double solvent overcomes homogeneous nucleation barriers (avoiding conventional nanocasting defects).
2.Performance mechanism:
- Enhanced mass diffusion: Interconnected 3D-ordered macropores in SOM-ZIF-8 are accessible from the external surface, facilitating bulky molecule (e.g., GFP) diffusion, unlike occluded macropores in PH-ZIF-8/M-ZIF-8.
- High stability: The robust single-crystalline framework of SOM-ZIF-8 has lower defect density than polycrystalline PH-ZIF-8, ensuring structural integrity during recycling.
Outlook:
This research innovatively realizes the construction of 3D-ordered macro-microporous MOF single crystals, breaking the bottleneck of traditional MOFs in diffusion-limited and bulky-molecule reactions. It provides a universal strategy for designing hierarchical porous single-crystalline materials, which is of great significance for promoting the application of MOFs in catalysis, separation, and other fields requiring efficient mass transfer and structural stability.
Ordered macro-microporous metal-organic framework single crystals
Authors: Kui Shen, Lei Zhang, Xiaodong Chen, Lingmei Liu, Daliang Zhang, Yu Han, Junying Chen, Jilan Long, Rafael Luque, Yingwei Li, Banglin Chen
DOI: 10.1126/science.aao3403
Link: https://www.science.org/doi/10.1126/science.aao3403
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

