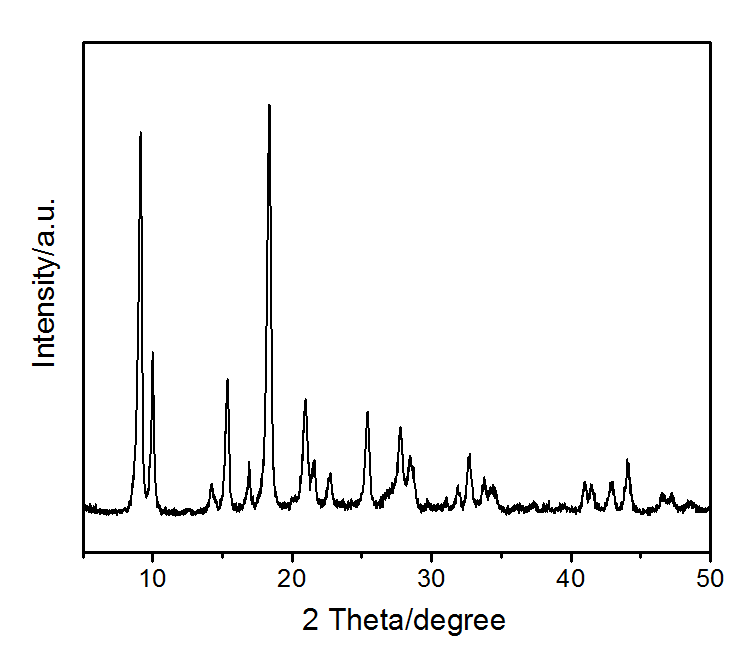
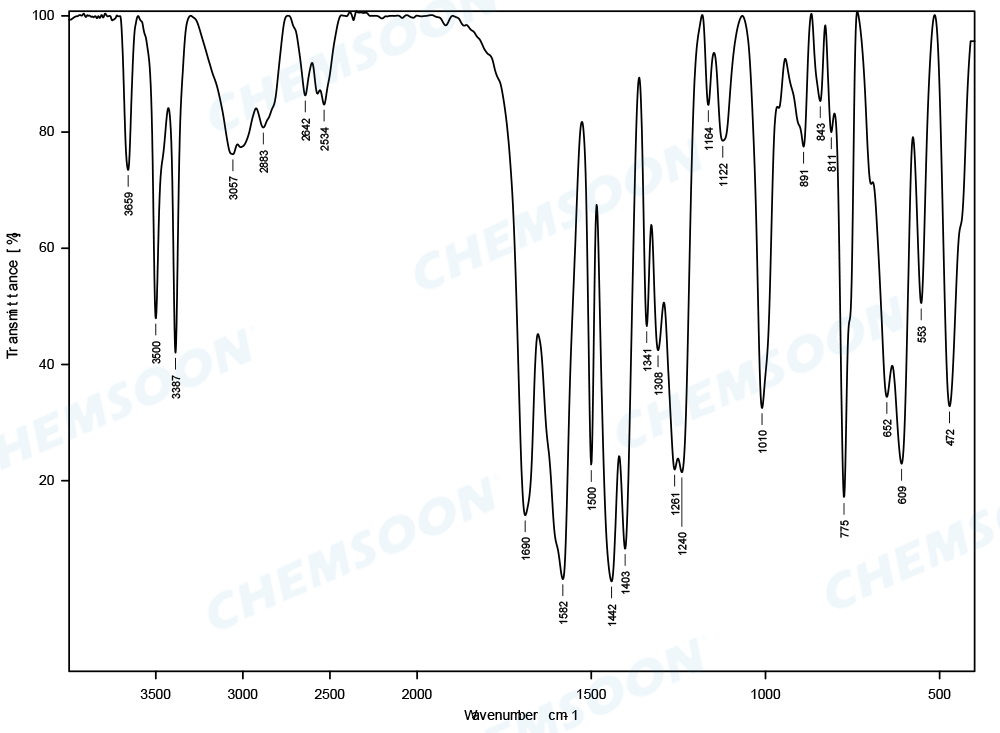
Product: NH2-MIL-53(Al)
Synonyms: NA
CAS:1134360-62-5
Basic Information
| Unit MF. | C8H5NO5Al | Unit MW. | 222.11 | ||
| Coordination Metal | Al | Linkers | 2-aminoterephthalic acid(CAS:10312-55-7) | ||
| Aperture | 0.8nm; 1.3nm | Pore volume | 0.5 cm3/g | ||
| Surface Area | BET Specific surface 950 m2/g | ||||
| Analog Structure | 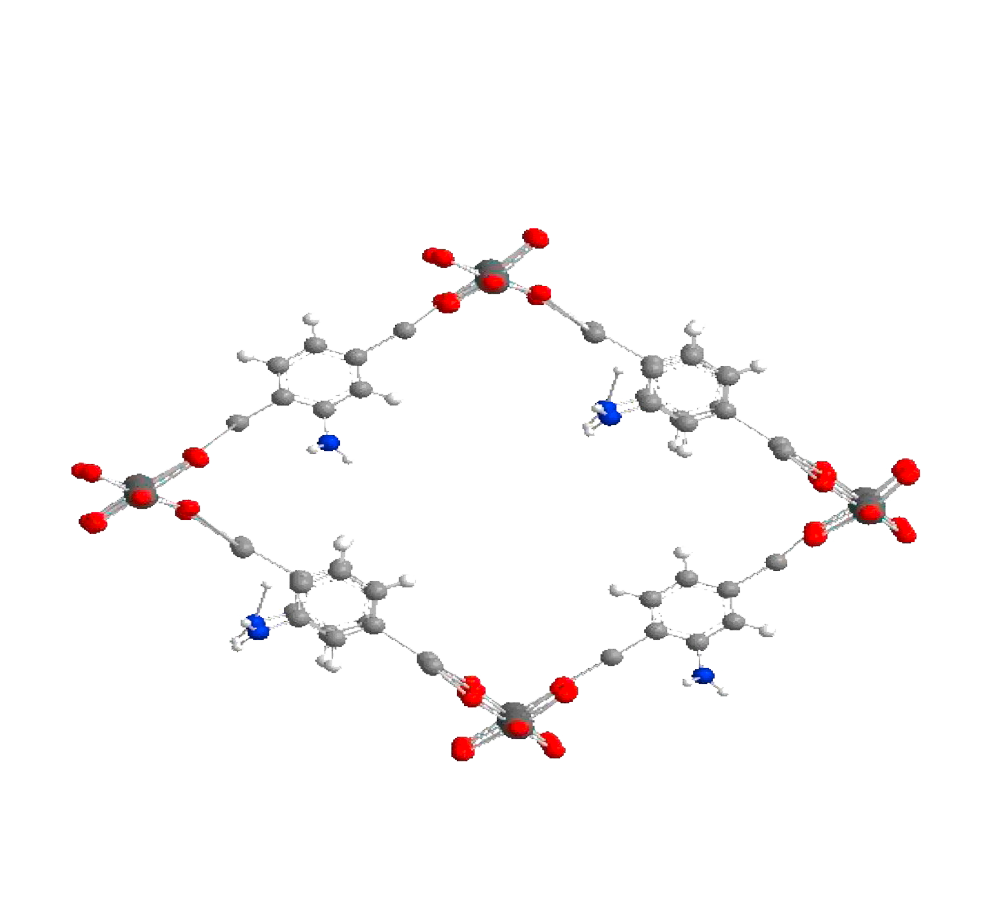 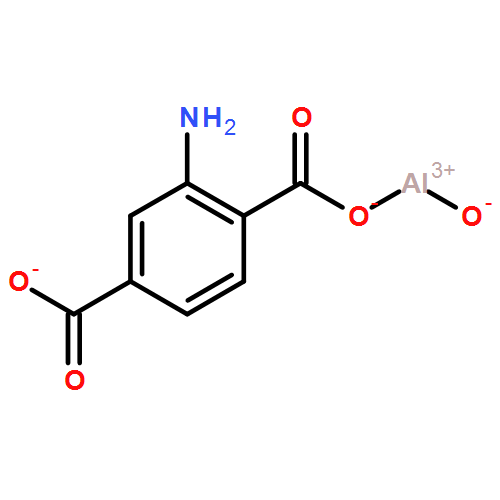 |
||||
Product Property
| Appearance | White Powder |  |
||
| Particle Size | 100nm or 500nm | |||
Stability
1) NH2-MIL-53(Al) is stable in air, stable in aqueous and alkaline solutions
2)High thermal stability, thermal decomposition temperature above 400 ° C
2)High thermal stability, thermal decomposition temperature above 400 ° C
Preservation
1) Keep sealed in dry and cool condition
2) It is recommended to activate for 3 hours at 150 degree in vacuum.
Other Features
Fluorescence:NA
Applications
1) Gas (such as carbon dioxide) and pollutant adsorption
2)Lewis acid catalyst
2)Lewis acid catalyst
Characterizations
References
1) Ahnfeldt, T., Gunzelmann, D., Loiseau, T., Hirsemann, D., Senker, J., Férey, G., Stock, N. Inorg. Chem.2009,48,7, 3057-3064; DOI: 10.1021/ic8023265 ; Synthesis and Modification of a Functionalized 3D Open-Framework Structure with MIL-53 Topology
2) Gascon, J.; Aktay, U.; Hernandez-Alonso, Maria D.; van K., Gerard P. M.; Kapteijn, F., Journal of Catalysis 2009, 261 (1), 75-87; DOI: 10.1016/j.jcat.2008.11.010 ; Amino-based metal-organic frameworks as stable, highly active basic catalysts;
3) Meng Jin, Zhao-Li Mou, Rui-Ling Zhang, Si-Si Liang, Zhi-Qi Zhang. Biosensors and Bioelectronics, 2017, 88, 447-454. DOI: 10.1016/j.bios.2016.12.022. An efficient ratiometric fluorescence sensor based on metal-organic frameworks and quantum dots for highly selective detection of 6-mercaptopurine;
4) Joseph G. Nguyen, Seth M. Cohen*;J. AM. CHEM. SOC., 2010, 132, 13; DOI:10.1021/ja100900c; Moisture-Resistant and Superhydrophobic Metal-Organic Frameworks Obtained via Postsynthetic Modification;
5) Wenchang Yin, Cheng-an Tao, Xiaorong Zou, Fang Wang, Tianlian Qu, Jianfang Wang; Nanomaterials, 2017, 7, 242; DOI:10.3390/nano7090242; The Tuning of Optical Properties of Nanoscale MOFs-Based Thin Film through Post-Modification;
6) Beatriz Zornoza, Alberto Martinez-Joaristi, Pablo Serra-Crespo, Carlos Tellez, Joaquin Coronas, Jorge Gascon, Freek Kapteijn; Chemical Communications, 2011, 47, 9522-9524; DOI :10.1039/c1cc13431k; Functionalized flexible MOFs as fillers in mixed matrix membranes for highly selective separation of CO₂ from CH₄ at elevated pressures;
7)Karen Leus, Thomas Bogaerts, Jeroen De Decker, Hannes Depauw, Kevin Hendrickx, Henk Vrielinck, Veronique Van Speybroeck, Pascal Van Der Voort; Microporous and Mesoporous Materials, 2016, 220; DOI:10.1016/j.micromeso.2015.11.055; Systematic study of the chemical and hydrothermal stability of selected “stable” Metal Organic Frameworks;
8)Chen Li, Zhenhu Xiong, Jinmiao Zhang, Chunsheng Wu; Journal of Chemical & Engineering Data, 2015, 60, 3414−3422; DOI:10.1021/acs.jced.5b00692; The Strengthening Role of the Amino Group in Metal−Organic Framework MIL-53 (Al) for Methylene Blue and Malachite Green Dye Adsorption;
9)Manuel Sánchez-Sánchez, Negash Getachew, Kenya Díaz, Manuel Díaz-García, Yonas Chebudeb, Isabel Díaz; Green Chem., 2015, 17, 1500-1509; DOI:10.1039/c4gc01861c; Synthesis of metal–organic frameworks in water at room temperature: salts as linker sources;
9)Manuel Sánchez-Sánchez, Negash Getachew, Kenya Díaz, Manuel Díaz-García, Yonas Chebudeb, Isabel Díaz; Green Chem., 2015, 17, 1500-1509; DOI:10.1039/c4gc01861c; Synthesis of metal–organic frameworks in water at room temperature: salts as linker sources;
10)Chunhua Li, Jianlong Wang, Li Zhu, Weixia Yang, Xie He, Sheliang Zhao, Xiaoshuo Zhang, Wenzhi Tang, Tianli Yue, Zhonghong Li; Journal of Agricultural and Food Chemistry, 2019, 67, 1277−1283; DOI:10.1021/acs.jafc.8b06253; Amino-Functionalized Al−MOF for Fluorescent Detection of Tetracyclines in Milk;
11)Alberto Martinez Joaristi, Jana Juan-Alcaniz, Pablo Serra-Crespo, Freek Kapteijn, Jorge Gascon;Cryst. Growth Des., 2012, 12, 3489-3498; DOI:10.1021/cg300552w; Electrochemical Synthesis of Some Archetypical Zn²⁺, Cu²⁺, and Al³⁺ Metal Organic Frameworks;