Home >
News > Systematic study of the chemical and hydrothermal stability of selected “stable” Metal Organic Frameworks
Systematic study of the chemical and hydrothermal stability of selected “stable” Metal Organic Frameworks
Summary:
The authors from Ghent University & Delft University: benchmarked the hydrothermal/chemical stability of nine “stable” MOFs (MIL-101(Cr), NH₂-MIL-101(Al), MIL-53(Al), NH₂-MIL-53, UiO-66, NH₂-UiO-66, UiO-67, ZIF-8, CuBTC), establishing a quantitative long-term (60 d) stability ranking that guides robust adsorbent/catalyst selection for harsh water, acid, base and H₂O₂ environments.
Background:
1. To address the problem, previous spot-check tests claimed individual MOFs as “water-stable”, yet lacked systematic comparison under identical long-term acid, base and oxidative stresses.
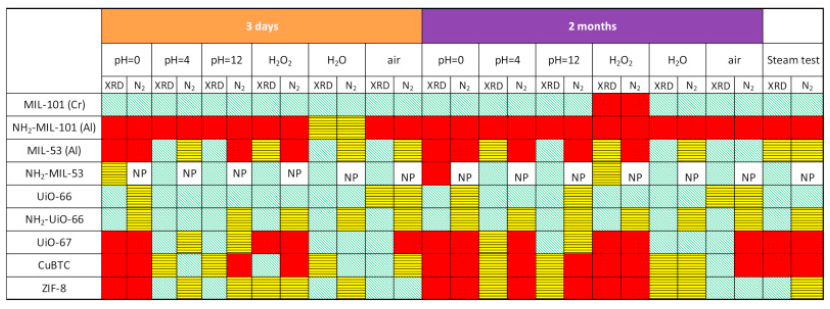
2. The authors proposed a standardized 3- & 60-day multi-stress protocol (pH 0, 4, 12; 5 wt % H₂O₂; 200 °C steam; air & water) and tracked both XRPD crystallinity and N₂-BET surface area to obtain an unbiased stability ladder.
Research Content:
1. Synthesis:
The authors synthesized the nine MOFs via reported hydro-/solvothermal routes (Cr/Al/Zr/Cu/Zn salts + terephthalic, 2-aminoterephthalic, BTC, HMe-Im linkers) without extra activation to mimic as-used samples.
2. Characterizations:
1) N₂ adsorption at −196 °C gave Langmuir areas (pristine 772–2395 m² g⁻¹) that were re-measured after each stress; pore-volume losses quantify degradation.
2) XRPD (Cu Kα) monitored phase purity; peak disappearance or emergence of NH₂-MIL-53, γ-AlO(OH), Cu₂OH(BTC) etc. was traced.
3) TGA verified acid-cleaning removal of occluded linker that artificially boosts MIL-101(Cr) area to >3200 m² g⁻¹.
3. Application:
The benchmark directly ranks MOFs for liquid-phase catalysis, oxidative detox, flue-gas dehydration, natural-gas sweetening and biomedical devices where long-term water/acid/base contact is inevitable.
4. Mechanism:
High-valent Cr³⁺/Zr⁴⁺ with carboxylates give strong M–O bonds and dense μ₃-oxo clusters, resisting hydrolysis; Al-based MOFs transform to denser MIL-53 when water relieves steric strain; Cu-paddle-wheels undergo Jahn–Teller axial aqua-coordination followed by SBU rupture; ZIF-8’s Zn–N(imidazolate) bonds survive base but are slowly carbonated by CO₂/H₂O/O₂.
Outlook:
The study delivers the first unified stability database and clear design rules: choose Cr-MIL-101 or UiO-66-type backbones for the harshest media, avoid NH₂-MIL-101(Al) and CuBTC in water, and always couple XRPD with surface-area assays to avoid false-positive “crystalline-but-clogged” materials.
Systematic study of the chemical and hydrothermal stability of selected “stable” Metal Organic Frameworks
Authors: Karen Leus, Thomas Bogaerts, Jeroen De Decker, Hannes Depauw, Kevin Hendrickx, Henk Vrielinck, Veronique Van Speybroeck, Pascal Van Der Voort
DOI: 10.1016/j.micromeso.2015.11.055
Link: https://www.sciencedirect.com/science/article/pii/S1387181115007209
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

