Product: CALF-20
Synonyms: Zn2(oxo)(trz)2
CAS:2615135-00-5
Basic Information
| Unit MF. | C6H4N6O4Zn2 | Unit MW. | 354.91 | ||
| Coordination Metal | Zn | Linkers | 1,2,4-三氮唑 1,2,4-triazole (CAS: 288-88-0) 草酸 oxalic acid (CAS: 144-62-7) | ||
| Aperture | 0.19-0.3 nm | Pore volume | |||
| Surface Area | no N2 adsorption | ||||
| Analog Structure |  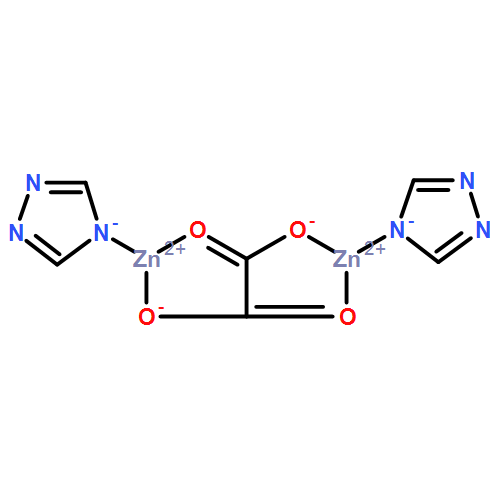 |
||||
Product Property
| Appearance | Off white Powders | |||
| Particle Size | irregular particles | |||
Stability
1) CALF-20 is stable in air, stable in aqueous and strong alkaline conditions. It is stable in humidity and acidic atmosphere.
2)High thermal stability, thermal decomposition temperature above 350 ° C
2)High thermal stability, thermal decomposition temperature above 350 ° C
Preservation
1) Keep sealed in dry and cool condition
2) It is recommended to activate for 12 hours at 120 degree in vacuum before Gas-Adsorption test
Other Features
Fluorescence:NA
Applications
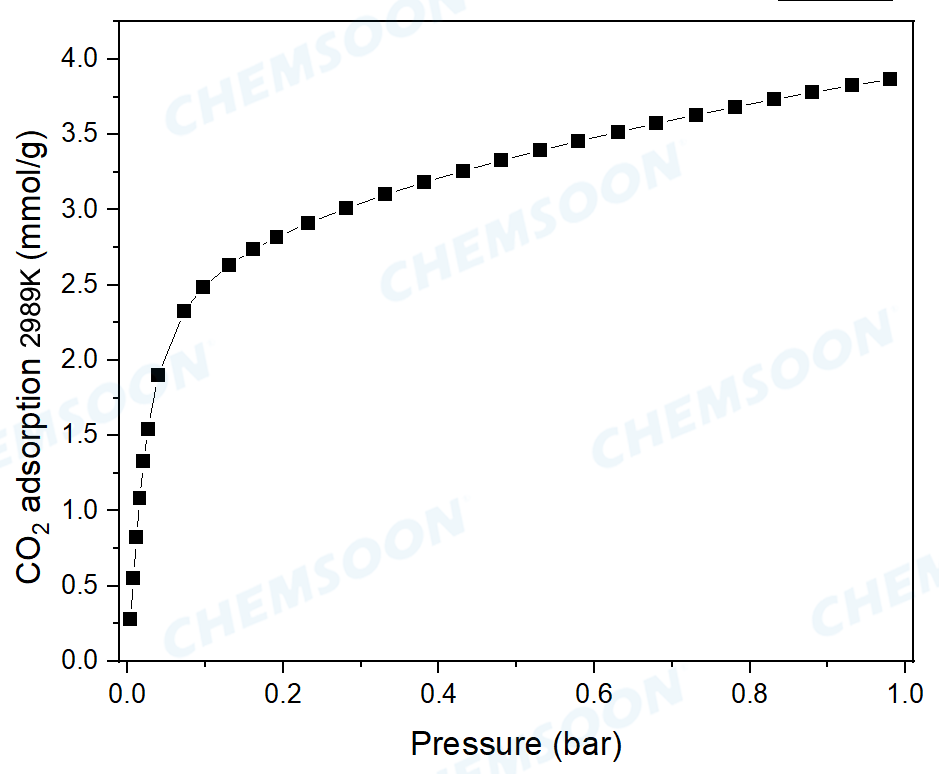
1) Prospective material for CO2 capture, CO2 uptake: 2.5mmol/g (P/P0=0.1, R.T.) and 3.5mmol/g (P/P0=1, R.T.)
2) With ultramicro pores below 0.3nm, it is prospective in separation of other industrial gas
2) With ultramicro pores below 0.3nm, it is prospective in separation of other industrial gas
Characterizations
References
1) Jian-Bin Lin; Tai T. T. Nguyen; Ramanathan Vaidhyanathan; Jake Burner; Jared M. Taylor; Hana Durekova; Farid Akhtar; Roger K. Mah; Omid Ghaffari-Nik; Stefan Marx; Nicholas Fylstra; Simon S. Iremonger; Karl W. Dawson; Partha Sarkar; Pierre Hovington; Arvind Rajendran; Tom K. Woo; George K. H. Shimizu; Science, 2021, 374, 1464–1469, DOI: 10.1126/science.abi7281 ; A scalable metal-organic framework as a durable physisorbent for carbon dioxide Capture
2) Rong Ji; Jianfang Wang; Caiping Sun; Xialin Chen; Chinese J. Struct. Chem. 2017,36(05), 769-774, DOI: 10.14102/j.cnki.0254-5861.2011-1412 ; Syntheses and Anti-lung Cancer Activities of Zn(Ⅱ) and Cu(Ⅱ) Complexes Based on Triazole and Tetrazole, respectively;
3) Sebastian Bette, Anastasia Sleptsova, Bettina V. Lotsch, Robert E. Dinnebier, Stefan Marx, Mahsa Loloei, Adebayo A. Adeleke, Nima Masoumifard, Ramanathan Vaidhyanathan; J. Am. Chem. Soc. 2025, 147, 29, 25662–25671; DOI:10.1021/jacs.5c06866; CO₂ and H₂O Sorption Induced Bulk-Phase Changes of CALF-20 Captured Using In Situ Laboratory X‑ray Powder Diffraction;
4) Federica Raganati, Mariangela Bellusci, Francesco Leardi, Francesca Varsano, Paola Ammendola; Chemical Engineering Journal, 2025; DOI:10.1016/j.cej.2025.159966; CALF-20 obtained by mechanochemical synthesis for temperature swing adsorption CO₂ Capture: A thermodynamic and kinetic study;
5) Yuto Higuchi, Miki Sugita, Saki Moriya, Takahiko Takewaki, Shunsuke Tanaka; Microporous and Mesoporous Materials, 2024; DOI:10.1016/j.micromeso.2024.113137; Rapid synthesis of metal-organic framework CALF-20 in H2O/methanol solution under room temperature and normal pressure;
6)Jon Hastings, Thomas Lassitter, Nicholas Fylstra, George K. H. Shimizu, T. Grant Glover*; Industrial & Engineering Chemistry Research, 2024, 63, 11544-11551; DOI:10.1021/acs.iecr.4c00373; Steam Isotherms, CO₂/H₂O Mixed-Gas Isotherms, and Single Component CO₂ and H₂O Diffusion Rates in CALF-20;
7)Jiangshang Su, Jiongfeng Li, Junhao Xu, Yongtao Li, Rou Zhang, Daofei Lv, Feng Xu, Junjie Peng, Xun Wang, Jian Yan, Zewei Liu, Xin Chen, Hongxia Xi, Qibin Xia; Industrial & Engineering Chemistry Research, 2024, 63, 18544-18551; DOI 10.1021/acs.iecr.4c02414; Cost-Effective Zinc-Based Metal−Organic Framework for Highly Efficient Methane Purification;