Product: UIO-66
Synonyms: NA
CAS:1072413-89-8
Basic Information
| Unit MF. | C48H24O32Zr6 | Unit MW. | 1660.03 | ||
| Coordination Metal | Zr | Linkers | Terephthalic acid (CAS: 100-21-0) | ||
| Pore Size | 0.8nm; 1.1nm | Pore volume | 0.5 cm3/g | ||
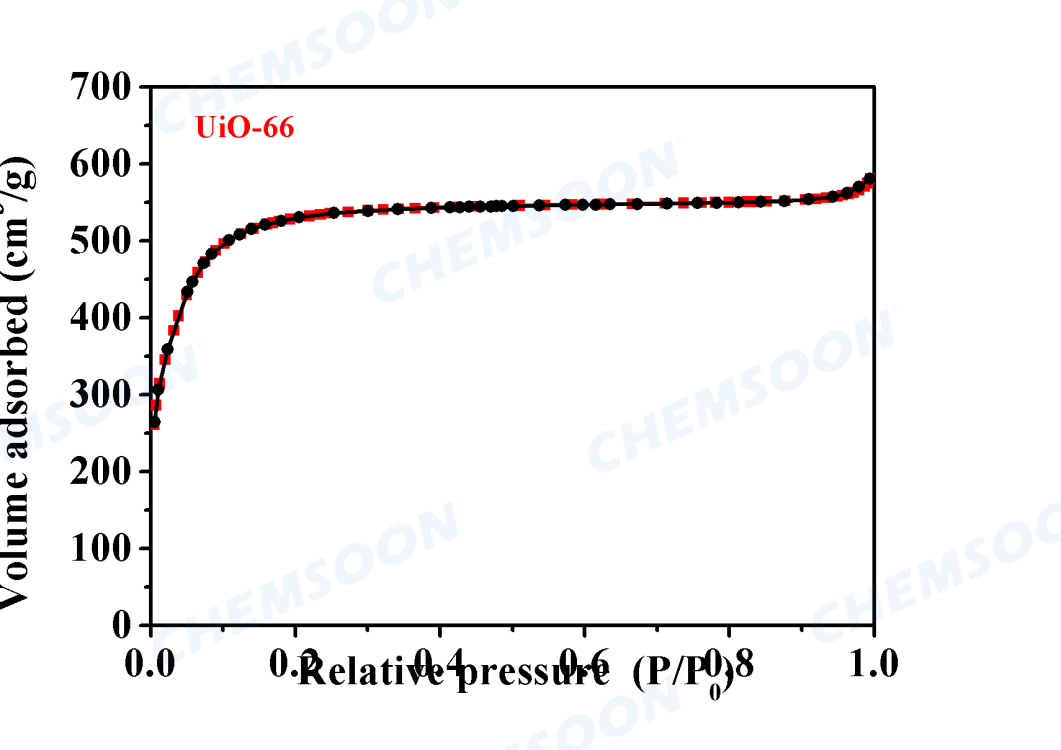
| Surface Area | BET Specific surface 1100 m2/g, | ||||
| Analog Structure |   |
||||
Product Property
| Appearance | White Powder |  |
||
| Particle Size | 200nm-1um | |||
Stability
1) UIO-66 is stable in air, stable in aqueous and acidic conditions(stable in PH 1-12)
2)High thermal stability, thermal decomposition temperature above 400 ° C
2)High thermal stability, thermal decomposition temperature above 400 ° C
Preservation
1) Keep sealed in dry and cool condition
2) It is recommended to degas for 12 hours at 120 degree in vacuum before gas-adsorption test.
2) It is recommended to degas for 12 hours at 120 degree in vacuum before gas-adsorption test.
Other Features
Fluorescence:NA
Applications
1) Gas (such as carbon dioxide) and pollutant adsorption
2) UIO-66 has very rigid framework, which is good carrier of catalyst.
2) UIO-66 has very rigid framework, which is good carrier of catalyst.
Characterizations
References
1) J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga, K. P. Lillerud, J. Am. Chem. Soc. 2008, 130, 13850-13851, DOI: 10.1021/ja8057953 ; A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability
2) S. J. Garibaya, S. M. Cohen, Chem. Commun., 2010,46, 7700-7702, DOI: 10.1039/C0CC02990D ; Isoreticular synthesis and modification of frameworks with the UiO-66 topology
3) P.Yang, F. Mao, Y. Li, Q. Zhuang, J. Gu, Chem. Eur. J. 2018, 24, 2962-2970, DOI : 10.1002/chem.201705020 ; Hierarchical Porous Zr‐Based MOFs Synthesized by a Facile Monocarboxylic Acid Etching Strategy;
4)Loredana Valenzano, Bartolomeo Civalleri, Sachin Chavan, Silvia Bordiga, Merete H. Nilsen, Søren Jakobsen, Karl Petter Lillerud, Carlo Lamberti; Chemistry of Materials, 2011, 23, 42; DOI :10.1021/cm1022882; Disclosing the Complex Structure of UiO-66 Metal Organic Framework: A Synergic Combination of Experiment and Theory;
5) Greig C. Shearer, Sachin Chavan, Silvia Bordiga, Stian Svelle, Unni Olsbye, Karl Petter Lillerud*; Chemistry of Materials, 2016, 28, 3749−3761; DOI:10.1021/acs.chemmater.6b00602; Defect Engineering: Tuning the Porosity and Composition of the Metal−Organic Framework UiO-66 via Modulated Synthesis;
6)Frederik Vermoortele, Bart Bueken, Gaëlle Le Bars, Ben Van de Voorde, Matthias Vandichel, Kristof Houthoofd, Alexandre Vimont, Marco Daturi, Michel Waroquier, Veronique Van Speybroeck, Christine Kirschhock, Dirk E. De Vos; Journal of the American Chemical Society, 2013, 135, 11465-11468; DOI:10.1021/ja405078u; Synthesis Modulation as a Tool To Increase the Catalytic Activity of Metal−Organic Frameworks: The Unique Case of UiO-66(Zr);
7) Claudia Gomes Silva, Ignacio Luz, Francesc X. Labres i Xamena, Avelino Corma; CHEMISTRY A EUROPEAN JOURNAL, 2010, 16, 11133-11138; DOI:10.1002/chem.200903526; Water Stable Zr–Benzenedicarboxylate Metal–Organic Frameworks as Photocatalysts for Hydrogen Generation;
8) 1) Jared B. DeCoste, Gregory W. Peterson, Himanshu Jasuja, T. Grant Glover, Yougui Huang, Krista S. Walton; Journal of Materials Chemistry A, 2013, 1, 5642-5650; DOI:10.1039/c3ta10662d; Stability and Degradation Mechanisms of Metal-Organic Frameworks containing the Zr₆O₄(OH)₄ Secondary Building Unit;
9)Gregory E. Cmarik, Min Kim, Seth M. Cohen, Krista S. Walton; Langmuir, 2012, 28, 15606-15613; DOI:10.1021/la3035352; Tuning the Adsorption Properties of UiO-66 via Ligand Functionalization;
10) Shixiong Li, Shengli Sun, Haizhen Wu, Chaohai Wei, Yun Hu; Catalysis Science & Technology, 2018, 8, 1696-1703; DOI:10.1039/c7cy02622f; Effects of electron-donating groups on the photocatalytic reaction of MOFs