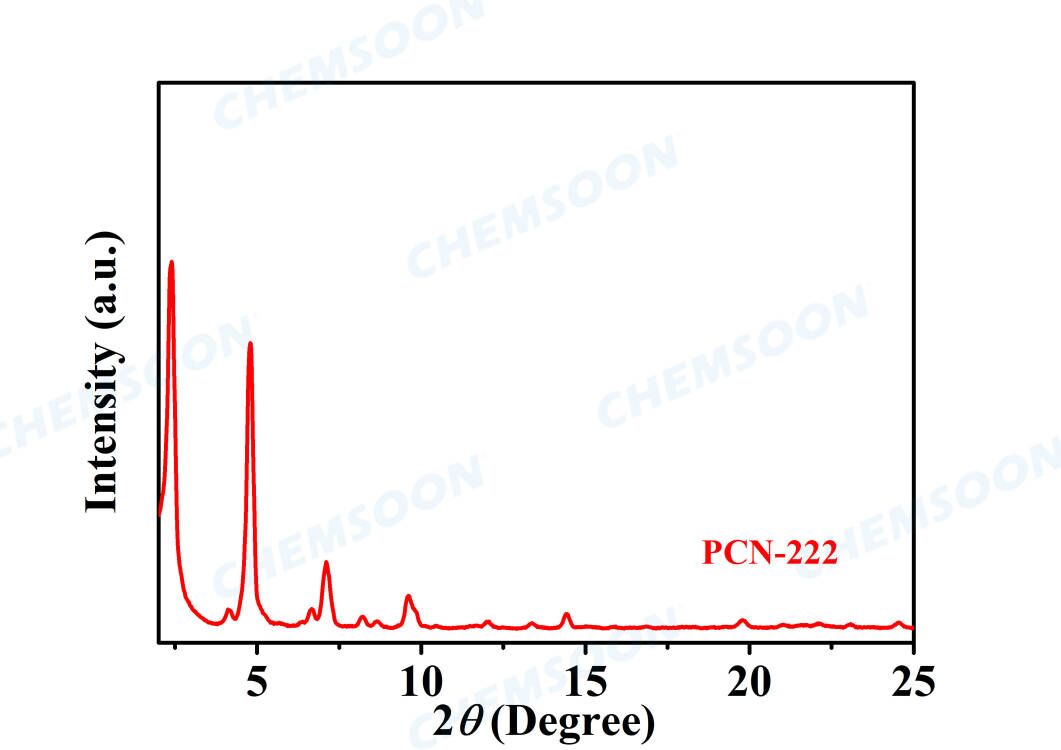
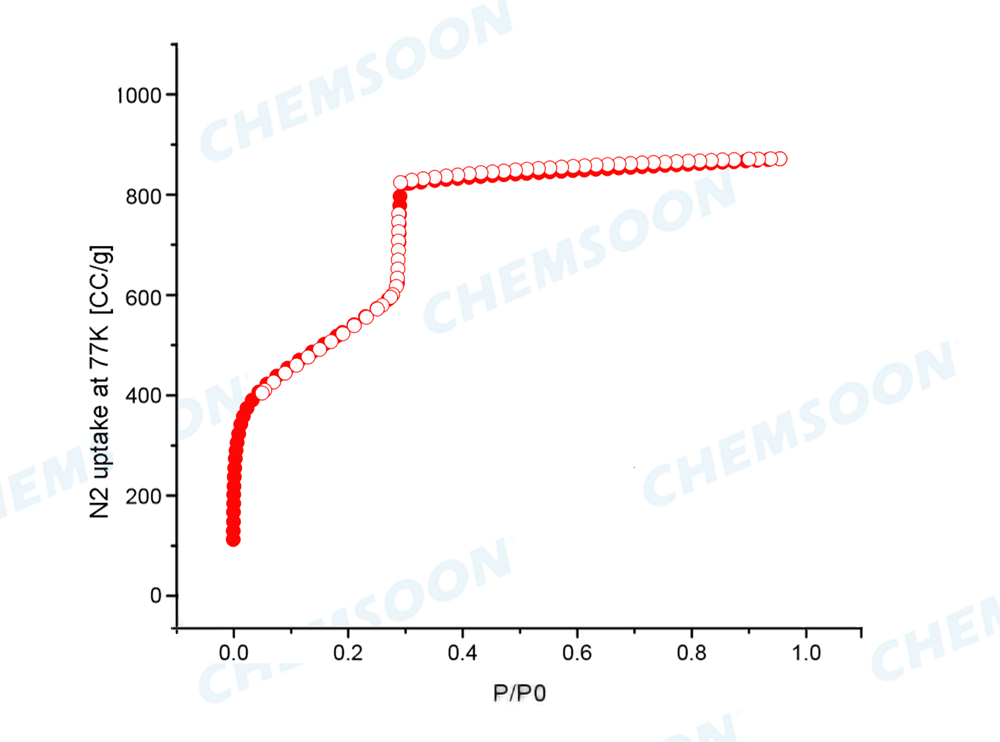
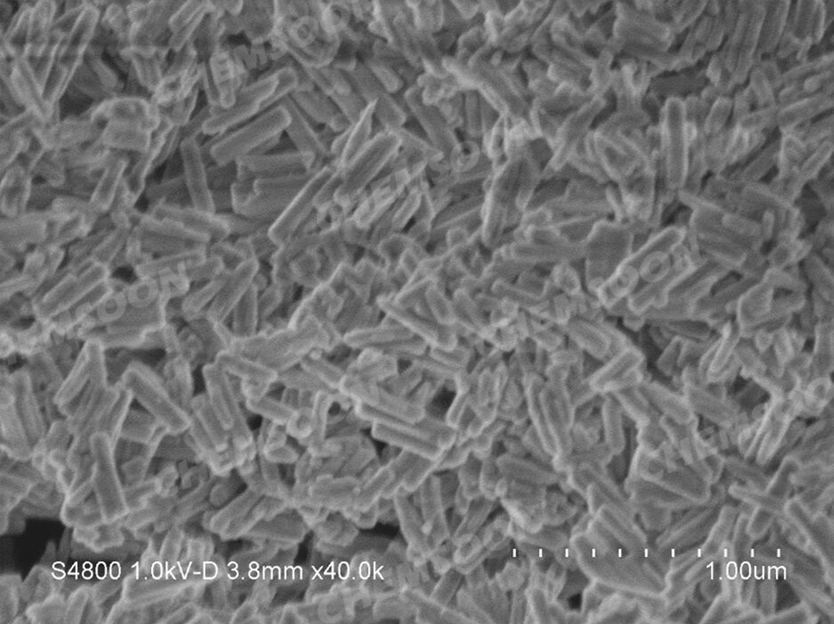
Product: PCN-222(H)
Synonyms: MOF-545
CAS:1403461-06-2
Basic Information
| Unit MF. | C96H52N8O32Zr6 | Unit MW. | 2376.81848 | ||
| Coordination Metal | Zr | Linkers | H2TCPP (CAS: 14609-54-2) | ||
| Pore Size | 3.7nm; 1.3 nm | Pore volume | 1.5 cm3/g | ||
| Surface Area | BET Specific surface 2000 m2/g | ||||
| Analog Structure | 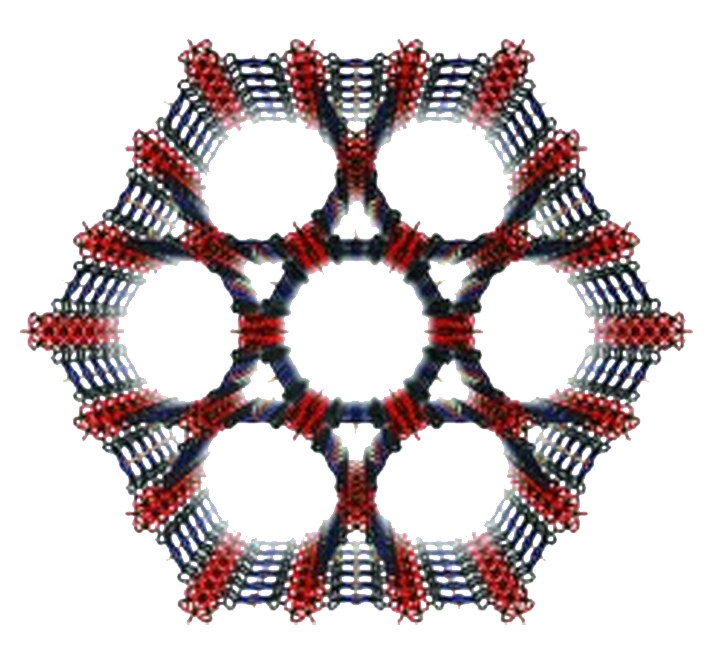 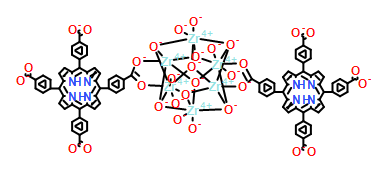 |
||||
Product Property
| Appearance | Dark Purple Powder |  |
||
| Particle Size | 2um*50nm needle particles; 200nm*20nm nanoparticles | |||
Stability
1) PCN-222 is stable in air, stable in aqueous and acidic conditions(stable in PH 1-11)
2)High thermal stability, thermal decomposition temperature above 400 ° C
2)High thermal stability, thermal decomposition temperature above 400 ° C
Preservation
1) Keep sealed in dry and cool condition
2) It is recommended to activate for 10 hours at 120 degree in vacuum.
2) It is recommended to activate for 10 hours at 120 degree in vacuum.
Other Features
Fluorescence: λex=413nm, λem=643nm
Applications
1) PCN-222 has rigid channel framework, which is good carrier of catalyst and poentail material for gas and pollutant adorption.
2) With a pore size up to 3.7nm, PCN-222 is a pontential material for adsorption of drug molecular and enzyme/peptide
3) PCN-222(H or M) is able to convert O2 to singlet oxygen under light condition. It could be used as photocatalyst in organic reaction or as biosensors.
Characterizations
References
1) D. Feng, Z.-Y. Gu, J.-R. Li, H.-L. Jiang, Z. Wei, and H.-C. Zhou, Angew. Chem. 2012, 124, 10453-10456, DOI: 10.1002/ange.201204475 ; Zirconium‐Metalloporphyrin PCN‐222: Mesoporous Metal–Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts
2) W. Morris, B. Volosskiy, S. Demir, F. Gándara, P. L. McGrier, H. Furukawa, D. Cascio, J. F. Stoddart, O. M. Yaghi, Inorg. Chem. 2012, 51, 6443-6445, DOI: 10.1021/ic300825s ; Synthesis, Structure, and Metalation of Two New Highly Porous Zirconium Metal–Organic Frameworks;
3) Yangyang Liu, Ashlee J. Howarth, Joseph T. Hupp, Omar K. Farha; Angewandte Chemie International Edition, 2015, 54, 9001-9005;Angewandte Chemie, 2015, 127, 9129-9133; DOI:10.1002/anie.201503741;Selective Photooxidation of a Mustard-Gas Simulant Catalyzed by a Porphyrinic Metal–Organic Framework;
4)Caiyun Xu, Hang Liu, Dandan Li, Ji-Hu Su, Hai-Long Jiang; Chemical Science, 2018, 9, 3152-3158; DOI:10.1039/c7sc05296k; Direct evidence of charge separation in a metal–organic framework: efficient and selective photocatalytic oxidative coupling of amines via charge and energy transfer;
5)Xu Chen, Yunhui Zhuang, Nakul Rampal, Rachel Hewitt, Giorgio Divitini, Christopher A. O’Keefe, Xiewen Liu, Daniel J. Whitaker, John W. Wills, Ravin Jugdaohsingh, Jonathan J. Powell, Han Yu, Clare P. Grey, Oren A. Scherman, David Fairen-Jimenez; Journal of the American Chemical Society, 2021, 143, 13557-13572; DOI:10.1021/jacs.1c03943; Formulation of Metal−Organic Framework-Based Drug Carriers by Controlled Coordination of Methoxy PEG Phosphate: Boosting Colloidal Stability and Redispersibility;
6)Hai-Qun Xu, Jiahua Hu, Dengke Wang, Zhaohui Li, Qun Zhang, Yi Luo, Shu-Hong Yu, Hai-Long Jiang;Journal of the American Chemical Society, 2015, 137, 13440-13443;DOI:10.1021/jacs.5b08773;Visible–Light Photoreduction of CO₂ in A Metal–Organic Framework: Boosting Electron–Hole Separation via Electron Trap States