Home >
News > Selective Photooxidation of a Mustard-Gas Simulant Catalyzed by a Porphyrinic Metal–Organic Framework
Selective Photooxidation of a Mustard-Gas Simulant Catalyzed by a Porphyrinic Metal–Organic Framework
Summary:
The authors fromNorthwestern University (USA) and King Abdulaziz University (Saudi Arabia) developed a porphyrinic metal–organic framework (PCN-222/MOF-545, free base: fb-1) with high porosity, wide pH stability, and efficient singlet oxygen (¹O₂) generation ability, achieving selective photooxidation of the mustard-gas simulant CEES to nontoxic CEESO in the application of chemical warfare agent (CWA) detoxification.
Background:
1. To address the detoxification of sulfur mustard (a highly toxic CWA still used in modern warfare), previous researchers explored three pathways: dehydrohalogenation (too slow for practical use), hydrolysis (limited by low solubility of sulfur mustard in water and slow intermediate formation), and oxidation (risk of overoxidation to toxic sulfone). Current oxidation methods using hydrogen peroxide or tert-butyl hydroperoxide often produce both nontoxic sulfoxide and toxic sulfone, failing to ensure selectivity.
2. The authors proposed an innovative method using PCN-222/MOF-545 as a heterogeneous photosensitizer, generating ¹O₂ via inexpensive commercial LEDs under mild conditions (room temperature, neutral pH) to selectively oxidize CEES to CEESO without toxic sulfone formation, and confirmed the catalyst’s reusability and structural stability.
Research Content:
1. Synthesis
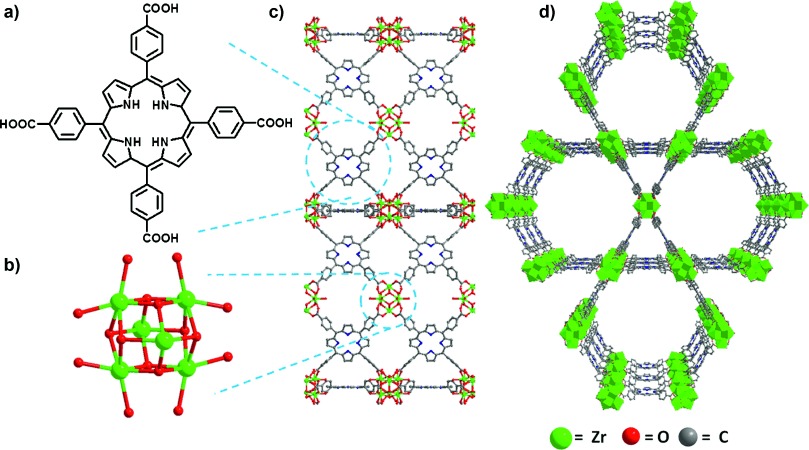
The authors synthesized free-base PCN-222/MOF-545 (fb-1) by reacting ZrCl₄, H₄TCPP (tetrakis(4-carboxyphenyl)porphyrin), and benzoic acid in DEF (N,N’-diethylformamide). The material consists of TCPP organic linkers and eightfold connected Zr₆ nodes, forming channels with diameters of 3.7 nm and 1.6 nm.
2. Characterizations
1.BET and pore size distribution: Nitrogen adsorption isotherms at 77K (Figure S2) confirmed the material’s activation; DFT-calculated pore size distribution (Figure S3) showed its porous structure, which enhances substrate-active site interactions.
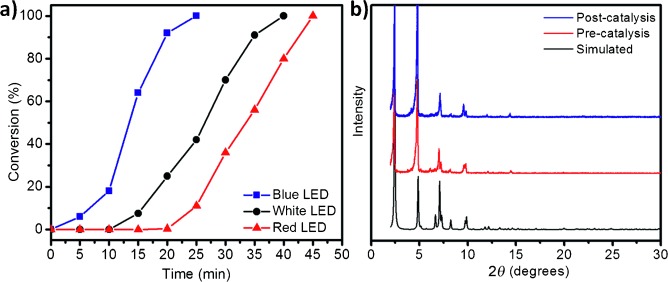
2.PXRD tests: Powder X-ray diffraction (Figure S4) showed that fb-1’s diffraction pattern matches the simulated one, confirming its crystallinity; post-catalysis PXRD (Figure 4b) proved the material retains its structure and crystallinity.
3.Other tests:
- ICP-OES (detection limit ~5 ppb) detected no Zr in the post-reaction filtrate (Table S1), confirming no metal leaching.
- NMR spectroscopy (¹H NMR, ¹³C NMR) confirmed no free porphyrin in the reaction solution, verifying heterogeneous catalysis.
- Singlet Oxygen Sensor Green (SOSG) fluorescence tests (Figure S20) confirmed fb-1 generates ¹O₂ with a rate constant of 1.5×10⁻³ s⁻¹ under red LED irradiation.
3. Application
The material was tested in the photooxidation of the mustard-gas simulant CEES:
- Under blue LED irradiation and O₂ atmosphere, 0.5 mol% fb-1 achieved full conversion of CEES to CEESO in 25 min (GC-MS and NMR confirmed no toxic CEESO₂ formation).
- The catalyst is reusable: four consecutive injections of CEES (0.2 mmol each) showed no loss of catalytic activity (Figure S6).
- The reaction works with air (instead of pure O₂) with a half-life of 21 min (Figure S7); blue LED (higher power) gives faster reactions than white or red LEDs, but all three ensure product selectivity (Figure 4a).
4. Mechanism
-¹O₂ generation mechanism: Under LED irradiation, the TCPP linker in fb-1 is excited (blue light excites Soret band, red light excites Q band, white light excites all visible bands). The excited triplet-state TCPP transfers energy to ground-state O₂ (³O₂), generating ¹O₂.
-Selective oxidation mechanism: ¹O₂, as a mild oxidant, only oxidizes the sulfide group in CEES to sulfoxide (CEESO) without further overoxidation to sulfone (CEESO₂). The porous 3D structure of fb-1 isolates porphyrin moieties (avoiding aggregation, a common issue with free porphyrins) and enhances CEES adsorption, accelerating catalytic kinetics.
Outlook:
This research innovatively applies porphyrinic MOFs to CWA detoxification, achieving selective, efficient, and mild oxidation of mustard-gas simulants. Its significance lies in:
- Providing a more realistic, convenient, and effective detoxification method (using low-cost LEDs, no toxic oxidants, mild conditions) compared to traditional methods.
- Verifying MOFs’ advantages (high porosity, tunable functionality, structural stability) in heterogeneous catalysis, laying a foundation for their application in gas-mask filtration systems and bulk sulfur mustard stockpile detoxification.
Selective Photooxidation of a Mustard-Gas Simulant Catalyzed by a Porphyrinic Metal–Organic Framework
Authors: Yangyang Liu, Ashlee J. Howarth, Joseph T. Hupp, Omar K. Farha
DOI: 10.1002/anie.201503741
Link: https://onlinelibrary.wiley.com/doi/10.1002/anie.201503741
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

