Home >
News > Direct evidence of charge separation in a metal–organic framework: efficient and selective photocatalytic oxidative coupling of amines via charge and energy transfer
Direct evidence of charge separation in a metal–organic framework: efficient and selective photocatalytic oxidative coupling of amines via charge and energy transfer
Summary:
The authors from the University of Science and Technology of China developed a porphyrinic metal–organic framework PCN-222 with semiconductor-like behavior and high porosity, achieving excellent activity, selectivity and recyclability in the application of visible-light-driven selective aerobic oxidative coupling of amines.
Background:
1. To address the issues of harsh reaction conditions (high temperature/pressure), low efficiency, difficult catalyst separation, unconfirmed oxidation products and low imine selectivity in amine oxidative coupling reactions, previous researchers used metal complex catalysts (requiring 373 K/5 atm) and heterogeneous semiconductor photocatalysts (unconfirmed oxidation products, aldehyde byproducts), yet problems like harsh conditions, low selectivity and unclear mechanism remained.
2. The authors proposed using PCN-222 with combined charge and energy transfer, realizing efficient amine oxidative coupling under ambient conditions and detecting key intermediates.
Research Content:
1.Synthesis:
- Synthesized TPPCOOMe by refluxing pyrrole and methyl 4-formylbenzoate in propionic acid, then acidified TPPCOOMe with KOH and HCl to get H₂TCPP.
- Synthesized PCN-222 by ultrasonic dissolving ZrOCl₂·8H₂O, H₂TCPP and CF₃COOH in DMF, then heating at 120℃ for ≥16 h.
2.Characterizations:
1) BET: PCN-222 has a BET surface area of 1659 m²/g; pore size distribution shows channels >3 nm.
2) SEM/TEM: Not mentioned in the literature.
3) Electrochemical/optical: UV-Vis shows absorption at 300-800 nm (Soret band 420 nm, Q bands 500-700 nm); cyclic voltammetry shows two oxidation peaks; Mott-Schottky plots confirm semiconductor properties.
3.Application:
Tested in amine oxidative coupling: benzylamine conversion reaches 100% in 1 h (CH₃CN, air, λ>420 nm); electron-donating substituted benzylamines react faster; 5 cycles maintain activity; TPPCOOMe only has 26.7% yield.
4.Mechanism:
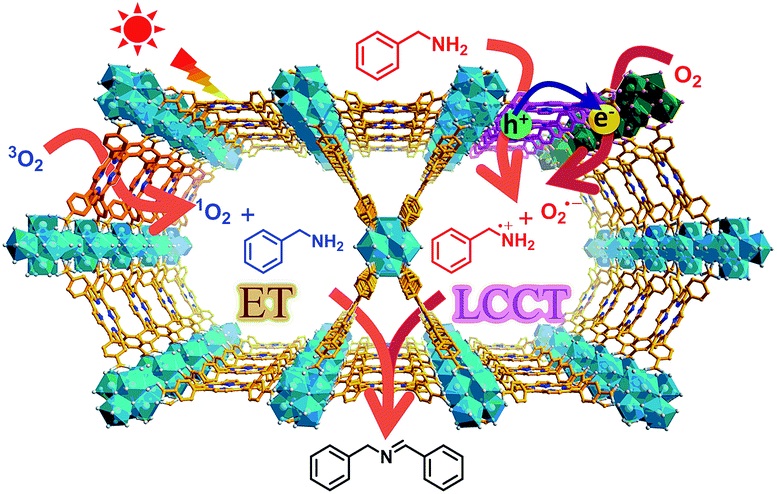
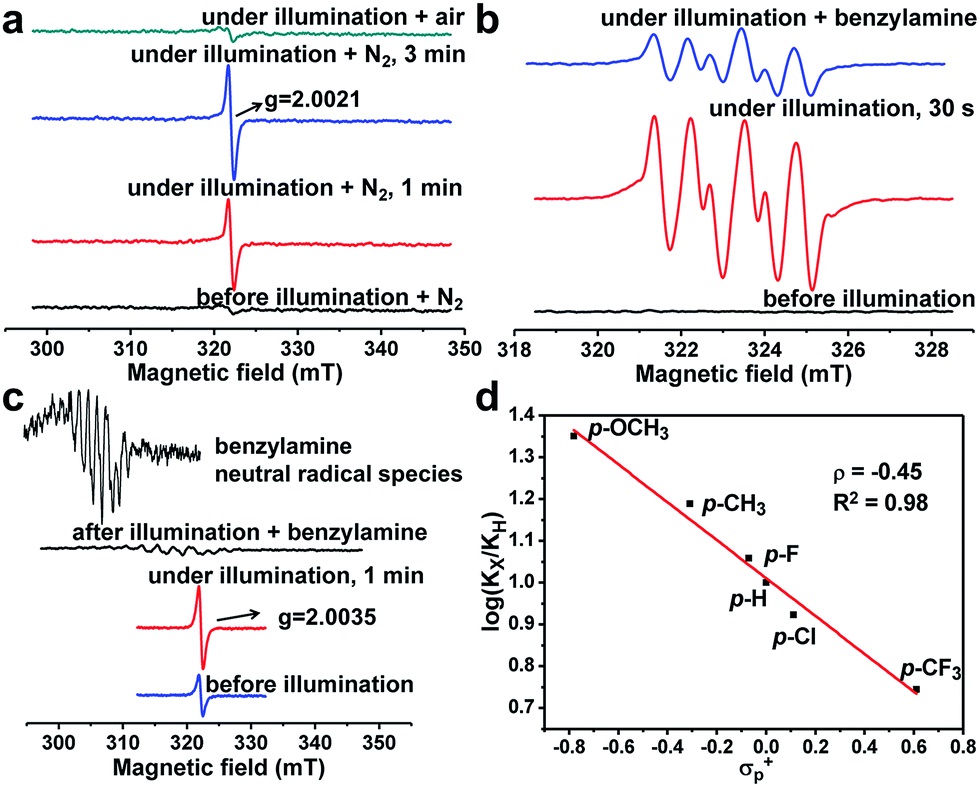
- Light excites PCN-222, triggering linker-to-cluster charge transfer (LCCT), generating Zr-oxo cluster oxygen-centered active sites (giso=2.0021) and porphyrin π-cation radicals.
- Electrons reduce O₂ to O₂⁻⁻ (detected by DMPO-ESR), holes oxidize amines to PhCH₂NH₂⁺·, then to PhCH·NH₂/PhCH₂NH· (ESR detected).
- Porphyrin generates ¹O₂ via energy transfer (4-oxo-TMP-ESR, g=2.0055), synergistically promoting imine formation.
Outlook:
This research confirms charge separation in MOFs, provides a clear amine oxidative coupling mechanism, and promotes MOF application in photocatalytic organic transformations.
Direct evidence of charge separation in a metal–organic framework: efficient and selective photocatalytic oxidative coupling of amines via charge and energy transfer
Authors: Caiyun Xu, Hang Liu, Dandan Li, Ji-Hu Su, Hai-Long Jiang
DOI: 10.1039/c7sc05296k
Link: https://pubs.rsc.org/en/content/articlelanding/2018/sc/c7sc05296k
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

