Home >
News > Formulation of Metal−Organic Framework-Based Drug Carriers by Controlled Coordination of Methoxy PEG Phosphate: Boosting Colloidal Stability and Redispersibility
Formulation of Metal−Organic Framework-Based Drug Carriers by Controlled Coordination of Methoxy PEG Phosphate: Boosting Colloidal Stability and Redispersibility
Summary:
The authors from the University of Cambridge (U.K.), Shanghai Institute of Technology (P.R. China), etc.: developed PEGylated Zr-based metal-organic framework (nanoMOF) materials with excellent colloidal stability, redispersibility, and delayed drug-release properties, achieving promising results in the application of drug delivery field.
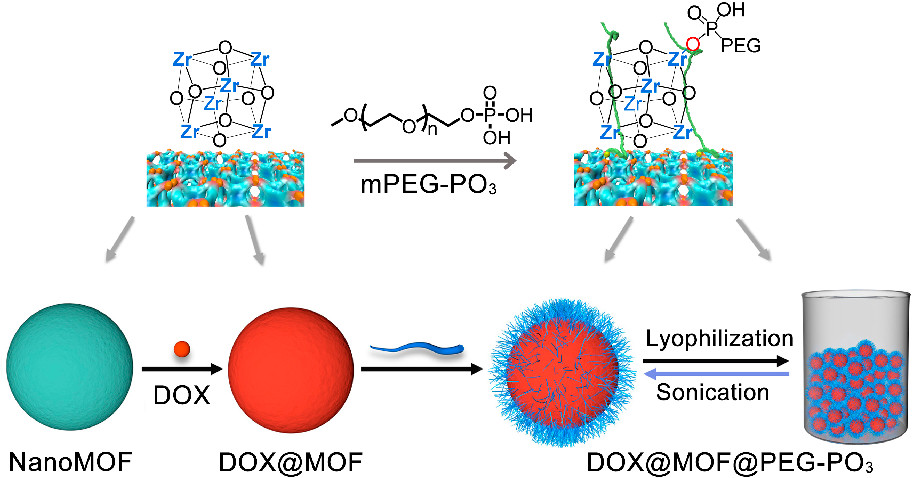
Background:
1. To address the barriers of nanoMOFs in clinical translation—such as tedious synthetic steps, toxic reagents, limited shelf life of nanoparticles in solution, and poor redispersibility after drying—previous researchers developed various postsynthetic modification strategies (e.g., click modulation with CuI catalyst, aryl radical grafting, phospholipid bilayer protection) to functionalize nanoMOFs. These strategies improved certain properties but still had issues like complex operations, introduction of toxic species, or failure to solve the redispersion problem.
2. The authors in this study proposed an innovative method: postsynthetic modification of Zr-based nanoMOFs with phosphate-functionalized methoxy polyethylene glycol (mPEG−PO₃) combined with lyophilization. This method solved the redispersion issue, enhanced colloidal stability, and achieved controlled drug release.
Research Content:
1. Synthesis
-mPEG−PO₃ synthesis: Prepared from poly(ethylene glycol) methyl ether (Mₙ=5k) and phosphorus oxychloride (POCl₃) via reaction in dry dichloromethane (DCM) with triethylamine, followed by dialysis and lyophilization.
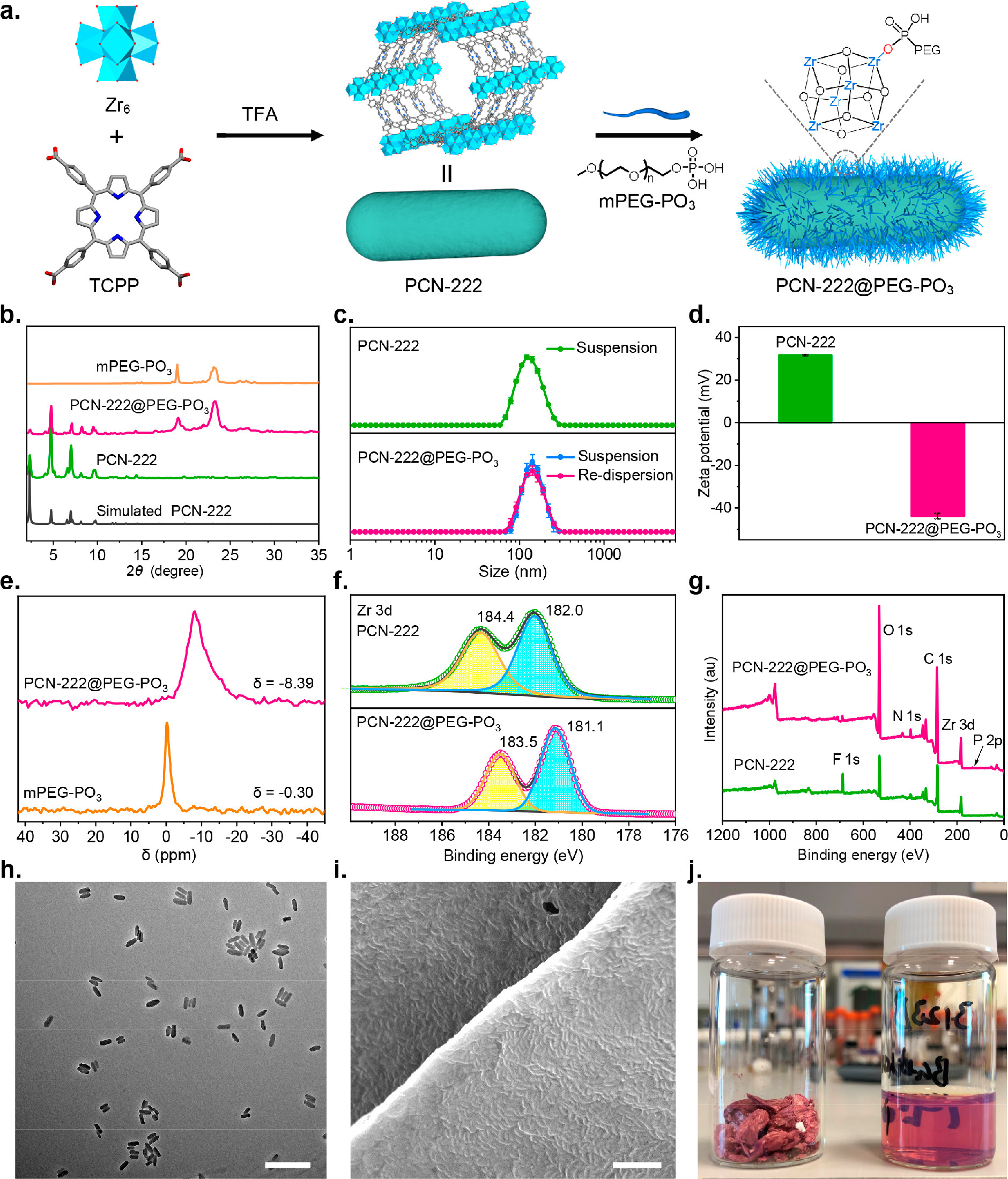
-Zr-based nanoMOFs synthesis: Synthesized PCN-222 (including Zr and Hf versions), UiO-66, MOF-808, NU-901, and PCN-128 via solvothermal reactions using corresponding linkers (e.g., TCPP for PCN-222) and Zr/Hf sources, with trifluoroacetic acid (TFA) as a modulator to control particle size.
-PEGylation and lyophilization: Mixed aqueous suspensions of nanoMOFs with mPEG−PO₃ solution (room temperature, 16h), centrifuged, dialyzed, and lyophilized (0.008 mBar, -70 °C) to obtain PEGylated nanoMOFs (MOF@PEG−PO₃); drug-loaded PEGylated nanoMOFs (DOX@MOF@PEG−PO₃) were prepared by loading doxorubicin (DOX) first, then PEGylation and lyophilization.
2. Characterizations
1.BET and pore size distribution:
- Parent PCN-222 had a BET area of 1151 m²/g; after PEGylation (16h), it decreased to 265 m²/g, and N₂ uptake at P/P₀=0.8 dropped from 526 to 129 cm³/g, indicating partial pore blocking by mPEG−PO₃.
- Other MOFs (e.g., UiO-66, MOF-808) showed similar trends: BET areas and pore volumes decreased after PEGylation, confirming partial porosity blockage.
2.SEM/TEM tests:
- PCN-222 had a rod-shaped morphology with an average size of 117.8 ± 12.9 nm; after PEGylation, TEM showed well-preserved morphology and monodispersity.
- Lyophilized MOF@PEG−PO₃ had low density, with nanoMOFs embedded in an mPEG matrix; ambient-dried samples aggregated, while lyophilized samples redispersed to maintain original hydrodynamic diameters (e.g., PCN-222@PEG−PO₃: ~129.7 nm vs. parent ~118.0 nm).
3.Other tests:
-PXRD: Confirmed maintained crystallinity of nanoMOFs after PEGylation, with new peaks at 2θ=19.0° and 23.2° from semicrystalline PEG.
-XPS: Zr 3d peaks of PCN-222 shifted from 184.4/182.0 eV to 183.5/181.1 eV after PEGylation, indicating Zr−O−P coordination; P 2p peaks confirmed mPEG−PO₃ incorporation.
-³¹P SSNMR: mPEG−PO₃ peak shifted from -0.30 ppm to -8.39 ppm after PEGylation, confirming interaction with the framework.
-Zeta potential: PCN-222 changed from +31.7 mV to -43.9 mV after PEGylation, improving colloidal stability.
3. Application
-Colloidal stability: Lyophilized MOF@PEG−PO₃ remained stable in water for up to 7 days (no significant size change) and in PBS (pH=7.4) for 36h, outperforming bare MOFs (aggregated within 1 day).
-Drug delivery (DOX as model drug):
- DOX loadings for bare nanoMOFs: 17.6 (UiO-66) - 23.2 (PCN-222) wt%; slightly decreased after PEGylation (13.4 - 20.2 wt%).
- Drug release: PEGylated nanoMOFs showed delayed release (e.g., PCN-128@PEG−PO₃ released <20% DOX in 6h vs. 52.5% for bare PCN-128 in PBS, pH=7.4).
-Cytotoxicity (HeLa cells):
- Bare PCN-128/PCN-222 showed cytotoxicity at high concentrations (e.g., 500 μg/mL, 72h); PEGylated counterparts had >80% cell viability at all tested concentrations, with reduced cytotoxicity.
-Cellular uptake:
- Flow cytometry showed faster uptake of bare PCN-128 (sharp increase in 6h) vs. PCN-128@PEG−PO₃ (slower, stable after 24h).
- Confocal microscopy confirmed internalization of both bare and PEGylated nanoMOFs; bare PCN-222 aggregated at cell surfaces, while PEGylated ones were more dispersed intracellularly.
4. Mechanism
-Two-step PEGylation:
1. Early stage (2h): mPEG−PO₃ binds to the external surface of positively charged nanoMOFs via electrostatic interaction (ICP-OES: 27.8 wt% loading, minimal pore blocking; BET=756 m²/g, N₂ uptake 91.6% of ideal value).
2. Later stage (4h+): After surface site saturation, excess mPEG−PO₃ infiltrates internal channels, blocking pores (loading plateaus at ~33 wt%, BET drops to ~250 m²/g).
-MD simulations: Confirmed mPEG−PO₃ is energetically favorable to anchor on Zr sites of PCN-222; early stage: PEG chains coil on the surface, with minimal pore infiltration; later stage: partial chain infiltration blocks pores.
-GCMC simulations: Calculated maximum DOX loadings (e.g., PCN-222: 56.5 wt%, NU-901: 34.6 wt%); DOX first adsorbs on framework walls, then fills pores, with preference for larger pores in mesoporous MOFs.
Outlook:
This research developed a mild, general PEGylation strategy for Zr-based nanoMOFs using mPEG−PO₃ and lyophilization. It solves the critical redispersion and shelf-life issues of MOF-based drug carriers, improves colloidal stability and biocompatibility, and enables controlled drug release. The work provides a feasible approach for long-term storage of MOF-drug composites, advancing the clinical translation of MOF-based drug delivery systems.
Formulation of Metal−Organic Framework-Based Drug Carriers by Controlled Coordination of Methoxy PEG Phosphate: Boosting Colloidal Stability and Redispersibility
Authors: Xu Chen, Yunhui Zhuang, Nakul Rampal, Rachel Hewitt, Giorgio Divitini, Christopher A. O’Keefe, Xiewen Liu, Daniel J. Whitaker, John W. Wills, Ravin Jugdaohsingh, Jonathan J. Powell, Han Yu, Clare P. Grey, Oren A. Scherman, David Fairen-Jimenez
DOI: 10.1021/jacs.1c03943
Link: https://pubs.acs.org/doi/10.1021/jacs.1c03943
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

