Home >
News > Stability and degradation mechanisms of metal–organic frameworks containing the Zr6O4(OH)4 secondary building unit
Stability and degradation mechanisms of metal–organic frameworks containing the Zr6O4(OH)4 secondary building unit
Summary:
The authors from SAIC, Edgewood Chemical Biological Center, and Georgia Tech systematically compared the hydro- and thermo-stability of four Zr6O4(OH)4-based MOFs (UiO-66, ‑NH2, ‑67 and a new bipyridine analog) and revealed clear linker-dependent degradation pathways, providing practical design rules for robust MOFs in humid or reactive media.
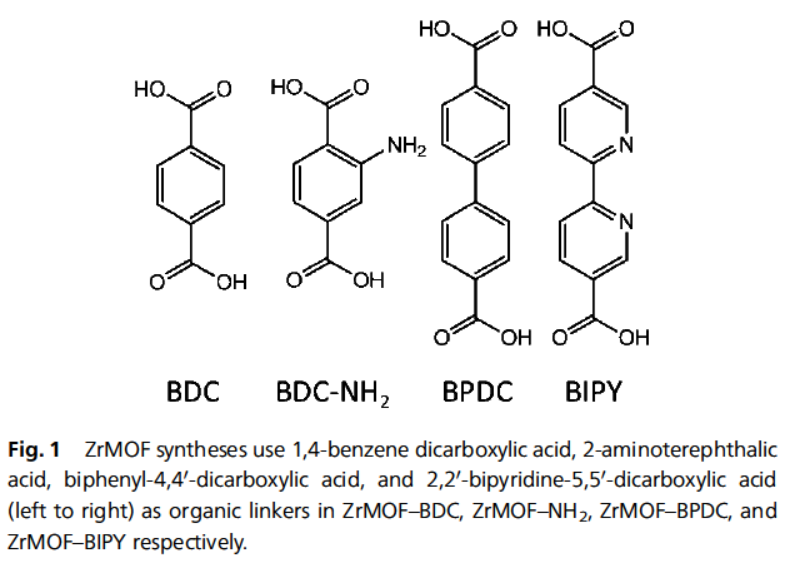
Background:
1. To overcome the poor water-resistance of common carboxylate MOFs, researchers have plasma-coated, carbon-coated or doped frameworks, yet these post-treatments add cost, lower porosity and may fail under prolonged liquid exposure.
2. The UiO series (Zr6O4(OH)4 SBU) is widely cited as “exceptionally stable”, but comparative data for linkers beyond terephthalate are scarce. The authors therefore proposed a quantitative, multi-technique screening protocol and uncovered striking linker-controlled instability.
Research Content:
1. Synthesis: Solvothermal reaction of ZrCl4 with terephthalic, 2-aminoterephthalic, biphenyl-4,4´-dicarboxylic or 2,2´-bipyridine-5,5´-dicarboxylic acid in DMF (120 °C, 24 h) gave ZrMOF-BDC, ‑NH2, ‑BPDC and ‑BIPY, respectively.
2. Characterizations:
1) BET: BDC 1080 m² g⁻¹; NH2 1005 m² g⁻¹; BPDC 2145 m² g⁻¹; BIPY 2385 m² g⁻¹—microporous (pore width ~0.8–1.2 nm).
2) PXRD confirms fcu topology; no linker decomposition peaks before exposure.
3) TGA shows onset decomposition 520 °C (BDC/BPDC), 480 °C (BIPY) and gradual 300–500 °C loss for NH2; in-situ heating PXRD tracks SBU collapse → tetragonal → monoclinic ZrO₂.
3. Application: 24 h immersion tests revealed BDC is stable to H₂O, MeOH, acetone, CHCl₃, pyridine and 0.1 M HCl (only NaOH destructs); BPDC loses crystallinity in H₂O, HCl, NaOH; BIPY is attacked even by MeOH/i-PrOH, losing 99 % surface area after 90 % RH vapor.
4. Mechanism: Steric & rotational strain in biphenyl/bipyridine linkers (energy maxima at 0° coplanarity) weaken Zr–O bonds; bipyridine additionally deprotonates protic solvents, generating aggressive alkoxide/hydroxide nucleophiles that cleave the SBU, whereas BDC’s compact pore hinders water clustering and retards hydrolysis.
Outlook:
The work demonstrates that “UiO stability” is linker-specific; increasing aromatic rings or introducing basic N-donors can sharply reduce chemical durability. These insights guide rational linker choice and surface engineering toward truly robust Zr-MOFs for catalysis, toxic gas filtration or humidity-swing separations.
Stability and degradation mechanisms of metal–organic frameworks containing the Zr6O4(OH)4 secondary building unit
Authors: Jared B. DeCoste, Gregory W. Peterson, Himanshu Jasuja, T. Grant Glover, You-gui Huang, Krista S. Walton
DOI: 10.1039/c3ta10662d
Link: https://pubs.rsc.org/en/content/articlelanding/2013/ta/c3ta10662d
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.
