Home >
News > Effects of electron-donating groups on the photocatalytic reaction of MOFs
Effects of electron-donating groups on the photocatalytic reaction of MOFs
Summary:
The authors from School of Environment and Energy, South China University of Technology and School of Biology and Biological Engineering, South China University of Technology developed MIL-101(Fe)-X and UiO-66-X (X=-OH, -NH₂, -COOH, -NO₂, -H) with adjustable electronegativity and stable structure, achieving high efficiency in photocatalytic degradation of phenanthrene in water.
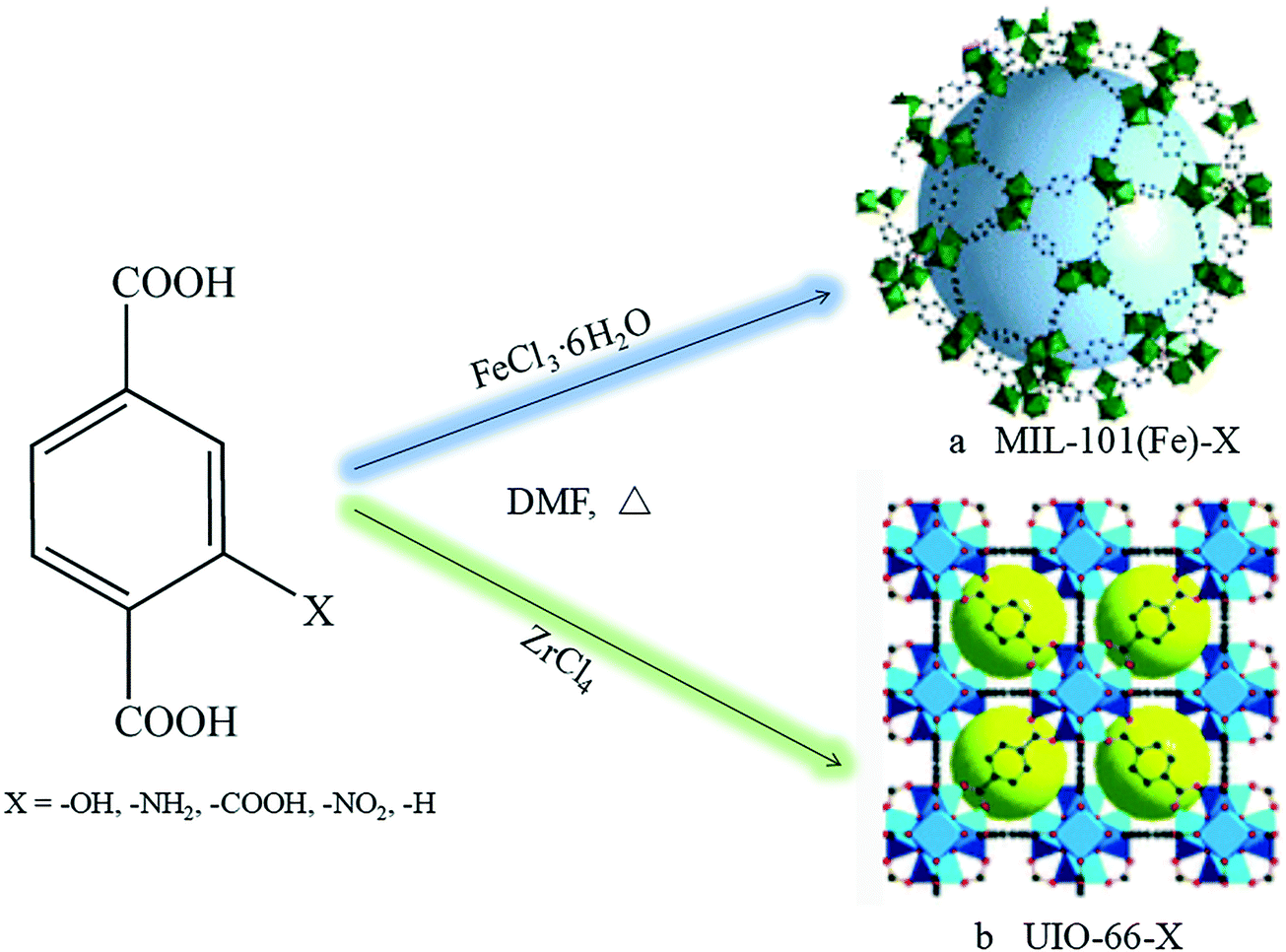
Background:
1. To address the problem of low efficiency of traditional photocatalysts (e.g., TiO₂ only responds to UV light, high electron-hole recombination rate) and unclear structure-activity relationship of MOFs in photocatalysis, previous researchers modified TiO₂ and studied MOFs, but failed to clarify the effect of electron-donating groups on MOFs' photocatalytic performance.
2. The authors proposed introducing different electron-donating groups into MIL-101(Fe) and UiO-66, obtained two series of MOFs, and confirmed that stronger electron-donating ability enhances photocatalytic efficiency.
Research Content:
1.Synthesis: The authors synthesized MIL-101(Fe)-X via solvothermal method (FeCl₃·6H₂O + corresponding ligands in DMF, 110℃ for 24h, washed and dried) and UiO-66-X via solvothermal method (ZrCl₄ + corresponding ligands in DMF, 120℃ for 24h, washed and dried), with yields 88%-95%.
2.Characterizations:
1) BET: MIL-101(Fe) has a specific surface area of 3753 m²/g; functionalized MIL-101(Fe)-X and UiO-66-X have lower specific surface areas.
2) SEM/TEM tests: Not mentioned in the documents.
3) Other tests: UV-vis DRS shows MIL-101(Fe)-X absorbs 220-800nm light; zeta potential (pH=7.08) of MIL-101(Fe)-OH is 22.84mV, UiO-66-OH is 15.31mV.
3.Application: The materials were tested in photocatalytic degradation of phenanthrene: MIL-101(Fe)-OH degrades 99.98% of phenanthrene in 120min, UiO-66-OH degrades 100% in 150min, better than TiO₂.
4.Mechanism: Electron-donating groups adjust MOFs' electronegativity via p-π conjugation, enhance ligand-to-metal charge transfer (LMCT), promote light absorption, reduce electron-hole recombination, and improve photocatalytic efficiency.
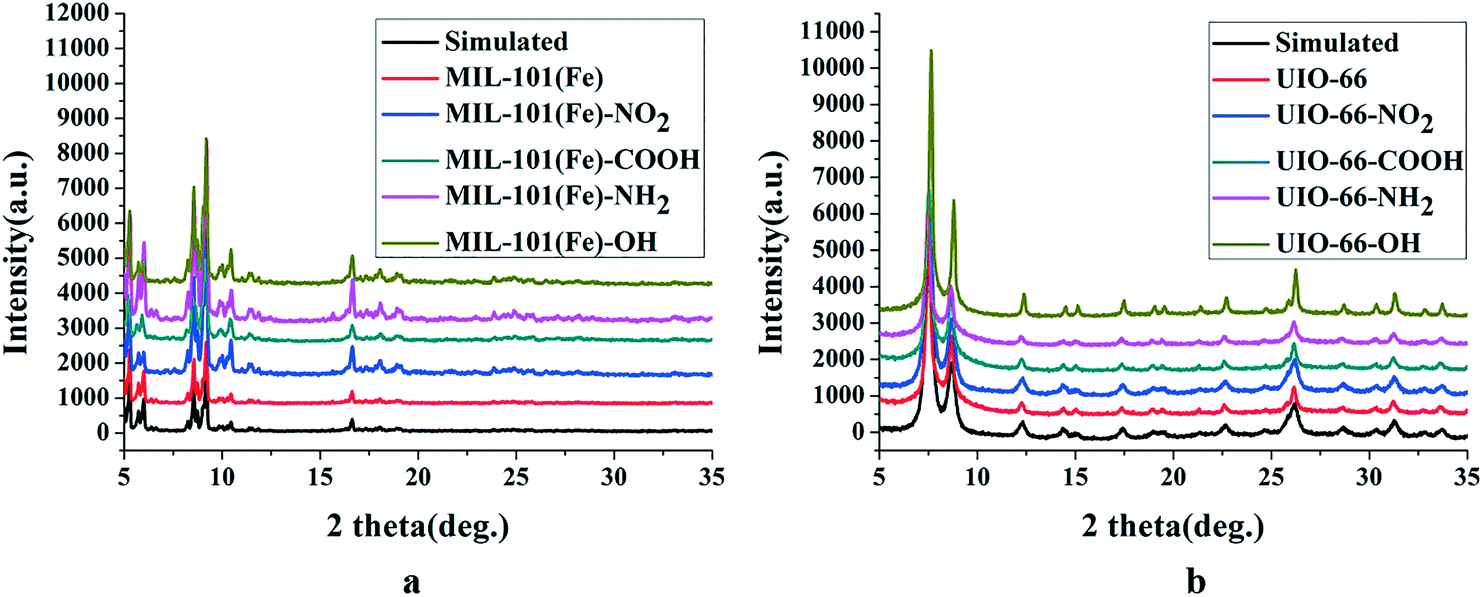
Outlook:
This research clarifies the effect of electron-donating groups on MOFs' photocatalysis, provides a molecular regulation strategy for high-efficiency photocatalysts, and has guiding significance for water organic pollutant treatment.
Effects of electron-donating groups on the photocatalytic reaction of MOFs
Authors: Shixiong Li, Shengli Sun, Haizhen Wu, Chaohai Wei, Yun Hu
DOI: 10.1039/c7cy02622f
Link: https://pubs.rsc.org/en/content/articlelanding/2018/cy/c7cy02622f
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

