Home >
News > Ultrahigh and Selective SO₂ Uptake in Inorganic Anion-Pillared Hybrid Porous Materials
Ultrahigh and Selective SO₂ Uptake in Inorganic Anion-Pillared Hybrid Porous Materials
Summary:
The authors from Zhejiang University (China), University of Amsterdam (Netherlands), National Institute of Standards and Technology (USA), and University of Texas at San Antonio (USA) developed inorganic anion-pillared hybrid porous materials (SIFSIX series, including SIFSIX-1-Cu, SIFSIX-2-Cu, etc.), which have ultrahigh and selective SO₂ adsorption characteristics, achieving excellent results in the application of gas purification (flue-gas desulfurization, natural-gas purification) field.
Background:
1. To address the problem of inefficient removal of low-concentration SO₂ (which poisons catalysts and inactivates CO₂ adsorbents) in gas purification, previous researchers used traditional flue-gas desulfurization (FGD) with limestone or organic solvents, achieving ~90-95% SO₂ removal, yet these technologies are energy-intensive and not efficient for deep desulfurization; existing MOFs also lack studies on selective SO₂ adsorption at low partial pressures and separation from CO₂.
2. The authors in this study proposed an innovative method of precisely tuning pore size and pore chemistry in SIFSIX metal-organic frameworks (MOFs) to realize selective recognition and dense packing of SO₂ clusters, obtaining ultrahigh SO₂ uptake capacity, unprecedented low-pressure SO₂ capacity, and record SO₂/CO₂ selectivity.
Research Content:
1. Synthesis
-SIFSIX-1-Cu: Dissolved 4,4’-bipyridine in ethylene glycol (338 K), added aqueous solution of Cu(BF₄)₂·xH₂O and (NH₄)₂SiF₆, heated at 65 °C for 3 h under stirring, filtered, washed with methanol, and exchanged with methanol for 3 days.
-SIFSIX-2-Cu: Layered ethanol solution of 4,4’-bipyridylacetylene onto ethylene glycol solution of CuSiF₆·xH₂O, obtained crystals after 2 weeks, exchanged with ethanol for 4 days.
-SIFSIX-2-Cu-i: Mixed methanol solution of 4,4’-bipyridylacetylene with aqueous solution of Cu(BF₄)₂·xH₂O and (NH₄)₂SiF₆, heated at 85 °C for 12 h, exchanged with methanol for 3 days.
-SIFSIX-3-Zn: Layered methanol solution of pyrazine onto methanol solution of ZnSiF₆·xH₂O, obtained colorless crystals after 2 days, exchanged with ethanol for 1 day.
-SIFSIX-3-Ni: Mixed methanol solution of NiSiF₆ and pyrazine, heated at 85 °C, obtained blue powder after 3 days, exchanged with ethanol for 3 days.
2. Characterizations
1.BET and pore size: BET surface area of fresh SIFSIX-2-Cu-i was 630 m²/g, and 620 m²/g after 75% humidity exposure; SIFSIX series have different pore sizes (SIFSIX-1-Cu with moderate pore size, SIFSIX-2-Cu-i with compact pore due to interpenetration, SIFSIX-3-Zn with small pore).
2.PXRD: SHIMADZU XRD-6000 and PANalytical X’Pert Pro diffractometers were used; Rietveld refinement confirmed SO₂ adsorption sites (e.g., SIFSIX-1-Cu·SO₂ with S···F distance 3.04 Å, SIFSIX-2-Cu-i·SO₂ with S···F distance 2.52 Å) and framework stability after SO₂ desorption/humidity exposure.
3.Other tests:
- TGA (Figure S15) showed thermal stability of SIFSIX-3-Zn and SIFSIX-3-Ni;
- Dynamic adsorption apparatus measured SO₂ uptake (weight change method);
- Gas chromatography (GC-2010 plus with TCD/FID) monitored breakthrough curves.
3. Application
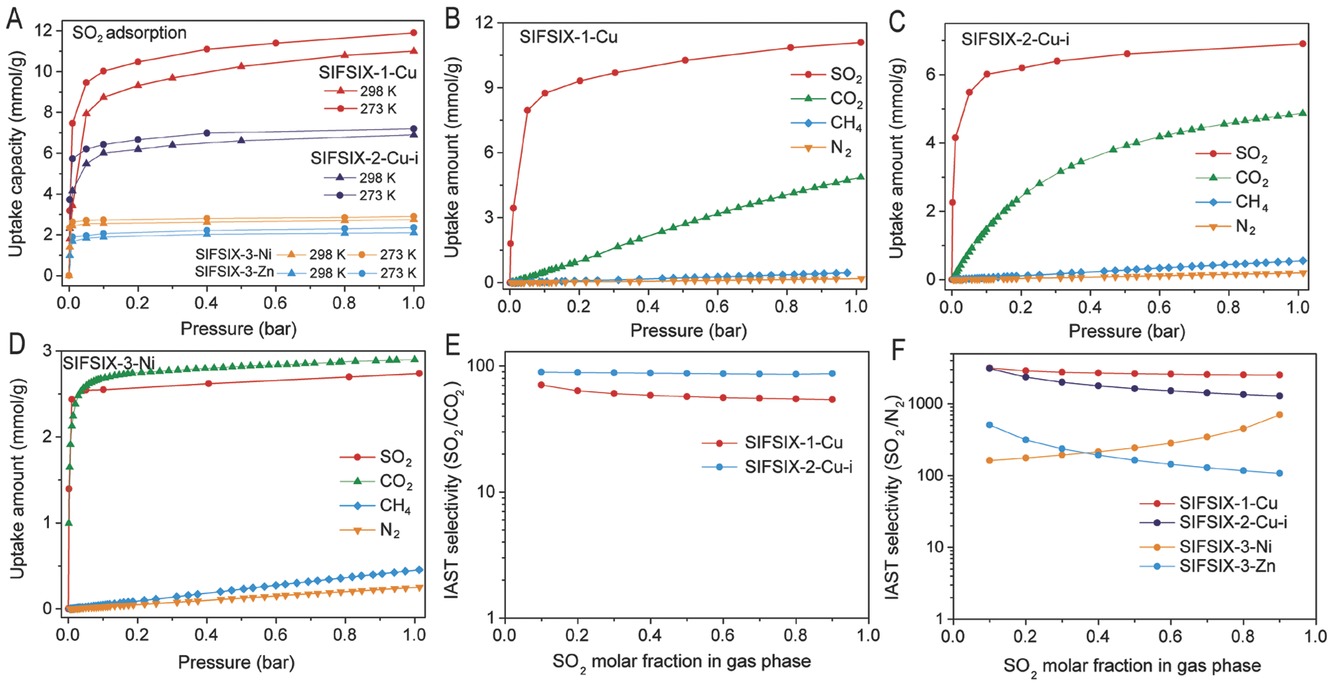
-SO₂ adsorption: At 298 K and 1.01 bar, SIFSIX-1-Cu achieved 11.01 mmol/g SO₂ uptake (highest under same conditions); SIFSIX-2-Cu-i had 2.31 mmol/g at 0.002 bar and 4.16 mmol/g at 0.01 bar (unprecedented low-pressure capacity).
-Gas separation: IAST calculations showed SIFSIX-2-Cu-i had SO₂/CO₂ selectivity 86-89 (record), SO₂/N₂ selectivity 1285-3103; SIFSIX-1-Cu had SO₂/CO₂ selectivity 54-70, SO₂/N₂ selectivity 2510-3145.
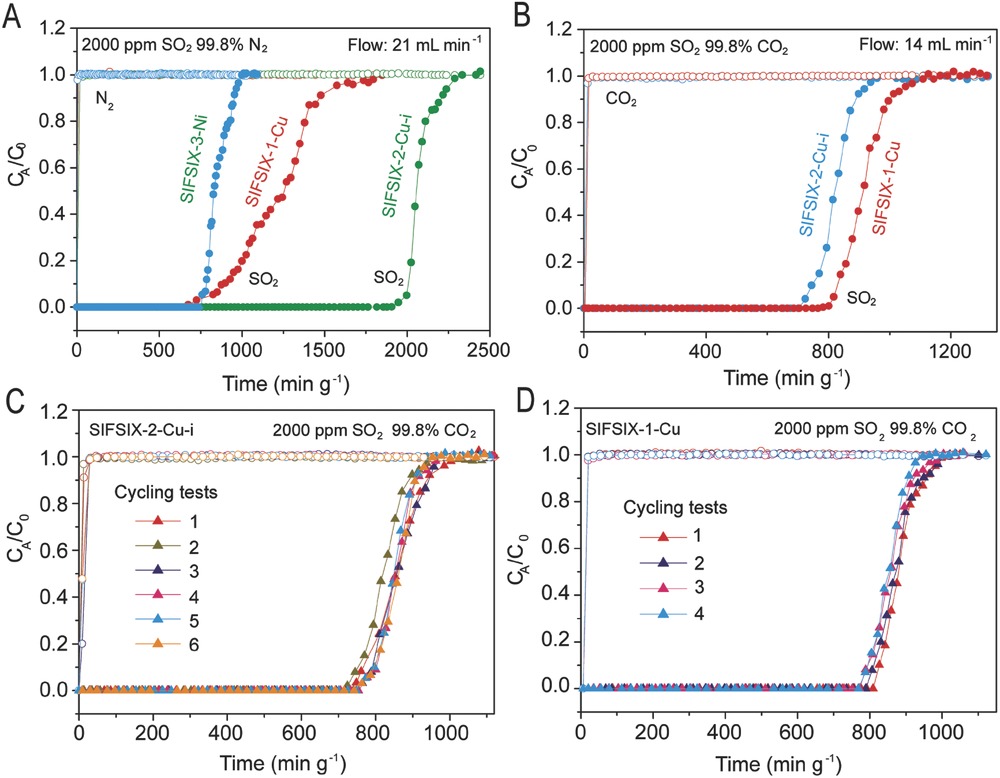
-Breakthrough tests: For 2000 ppm SO₂/N₂ mixture, SIFSIX-2-Cu-i had breakthrough time 1800 min/g; for 2000 ppm SO₂/CO₂ mixture, it realized efficient separation; 6 cycles for SIFSIX-2-Cu-i and 4 cycles for SIFSIX-1-Cu showed no performance decline.
-Regeneration and stability: Fully regenerated by He flow at 313 K; stable after humidity exposure (75% for 1 day) and SO₂ corrosion.
4. Mechanism
-Host-guest interactions: SO₂ is adsorbed via Sᵟ⁺···Fᵟ⁻ electrostatic interactions (S···F distance 2.44-3.60 Å, shorter than van der Waals sum) and Oᵟ⁻···Hᵟ⁺ dipole-dipole interactions (O···H distance 2.39-3.58 Å) with aromatic linkers, grasping every SO₂ atom.
-Guest-guest interactions: Adjacent SO₂ molecules have S···O distance 3.2-4.07 Å (comparable to liquid SO₂), forming dense SO₂ clusters and promoting secondary adsorption.
-DFT calculations: Quantum-Espresso package (GGA-PBE, van der Waals correction) showed SO₂ adsorption energy (ΔE) in SIFSIX-1-Cu (50.3 kJ/mol) and SIFSIX-2-Cu-i (50.2 kJ/mol) was much higher than CO₂ (31.1-35.7 kJ/mol) and N₂ (15.7-25.2 kJ/mol), confirming stronger SO₂ interactions.
-Pore size effect: Moderate pore size (SIFSIX-1-Cu, SIFSIX-2-Cu-i) balances host-guest/guest-guest interactions for dense SO₂ packing; too large (SIFSIX-2-Cu) or small (SIFSIX-3-Zn) pores reduce capacity.
Outlook:
This research realizes selective recognition and dense packing of SO₂ clusters in MOFs for the first time, setting new benchmarks for SO₂ capture (ultrahigh capacity, low-pressure performance, high selectivity). It provides a design strategy for porous materials (tuning pore chemistry/size) and is instructive for efficient SO₂ removal in flue gas/natural gas, as well as other gas-purification processes.
Ultrahigh and Selective SO₂ Uptake in Inorganic Anion-Pillared Hybrid Porous Materials
Authors: Xili Cui, Qiwei Yang, Lifeng Yang, Rajamani Krishna, Zhiguo Zhang, Zongbi Bao, Hui Wu, Qilong Ren, Wei Zhou, Banglin Chen, Huabin Xing
DOI: 10.1002/adma.201606929
Link: https://onlinelibrary.wiley.com/doi/10.1002/adma.201606929
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

