Home >
News > Shaping of ultrahigh-loading MOF pellet with a strongly anti-tearing binder for gas separation and storage
Shaping of ultrahigh-loading MOF pellet with a strongly anti-tearing binder for gas separation and storage
Summary:
The authors from the Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University developed ultrahigh-loading MOF pellets (SIFSIX-3-Ni, SIFSIX-2-Cu-i, GeFSIX-2-Cu-i, TIFSIX-2-Cu-i, etc.) using polyvinyl butyral (PVB) as a strongly anti-tearing binder. These pellets have characteristics of up to 95% MOF loading, negligible C₂H₂ adsorption loss, and enhanced C₂H₂/C₂H₄ selectivity, achieving excellent results in C₂H₂/C₂H₄ separation, SO₂ adsorption, and CO₂ capture in the field of gas separation and storage.
Background:
1. To address the issue that MOF powders are brittle, non-thermoplastic, and insoluble, hindering their industrial application, previous researchers used binders like PVA, sucrose, and alumina sol for MOF pelletization. For example, MIL-53(Al) with PVA binder had a 32% BET surface area reduction, and Zr-MOF with sucrose binder had a 34.8% H₂ storage capacity loss—these methods failed to balance high MOF loading and retention of adsorption performance, and no studies focused on anion-pillared ultramicroporous MOF pelletization.
2. The authors proposed an innovative method using PVB as a binder for MOF pelletization. PVB has strong adhesion, anti-tearing properties, and good compatibility with MOFs, enabling ultrahigh MOF loading (up to 95%) while maintaining or enhancing gas adsorption/separation performance, and the method is applicable to diverse MOFs.
Research Content:
1. Synthesis
-MOF powder synthesis:
- SIFSIX-3-Ni: Mixed methanol solution of (NH₄)₂SiF₆, Ni(BF₄)₂, and pyrazine, heated at 85 °C for 3 days, washed, methanol-exchanged, dried, and degassed.
- SIFSIX-2-Cu-i: Mixed methanol solution of 4,4’-bipyridylacetylene with aqueous solution of Cu(BF₄)₂·xH₂O and (NH₄)₂SiF₆, heated at 85 °C for 12 h, methanol-exchanged, dried, and degassed.
- GeFSIX-2-Cu-i: Mixed methanol solution of 4,4’-bipyridylacetylene with aqueous solution of Cu(BF₄)₂·xH₂O and (NH₄)₂GeF₆, heated at 65 °C for 12 h, methanol-exchanged, dried, and degassed.
- TIFSIX-2-Cu-i: Mixed methanol solution of 4,4’-bipyridylacetylene with aqueous solution of Cu(BF₄)₂·xH₂O and (NH₄)₂TiF₆, heated at 85 °C for 12 h, methanol-exchanged, dried, and degassed.
- HKUST-1, Mg-MOF-74, MIL-101-Cr: Synthesized via solvothermal methods with corresponding precursors (e.g., Mg-MOF-74 from Mg(NO₃)₂·6H₂O and 2,5-dihydroxyterephthalic acid), followed by solvent exchange and degassing.
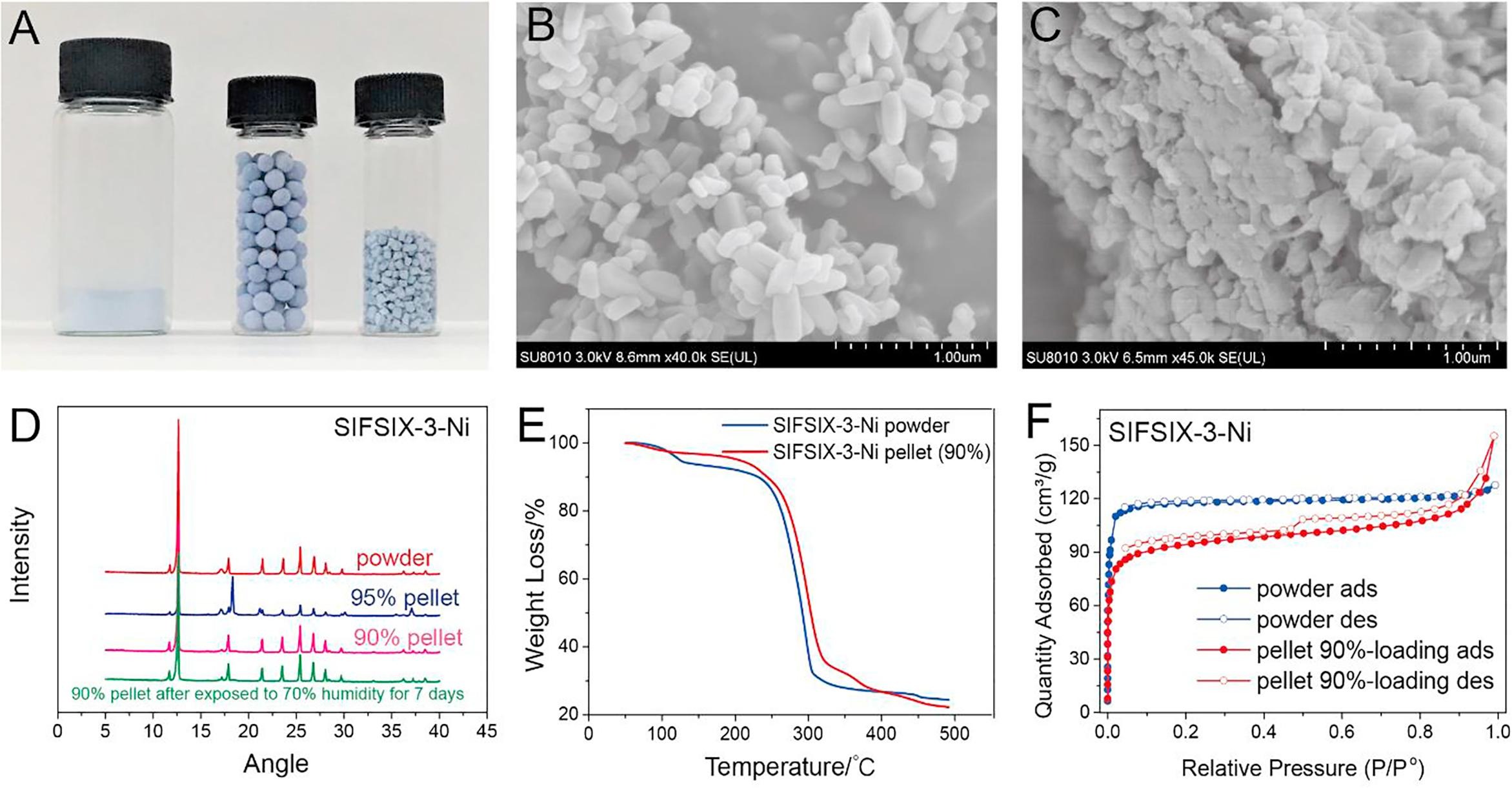
-MOF pellet preparation: Prepared 8 wt% PVB/ethanol solution; added the solution to MOF powder in small portions (e.g., 0.31 g solution to 0.5 g SIFSIX-3-Ni for 95% loading), shaped into 1–2 mm cubic pellets by extrusion, heated at 60 °C for 24 h (Mg-MOF-74 and HKUST-1 molded under N₂).
2. Characterizations
1.BET and pore size:
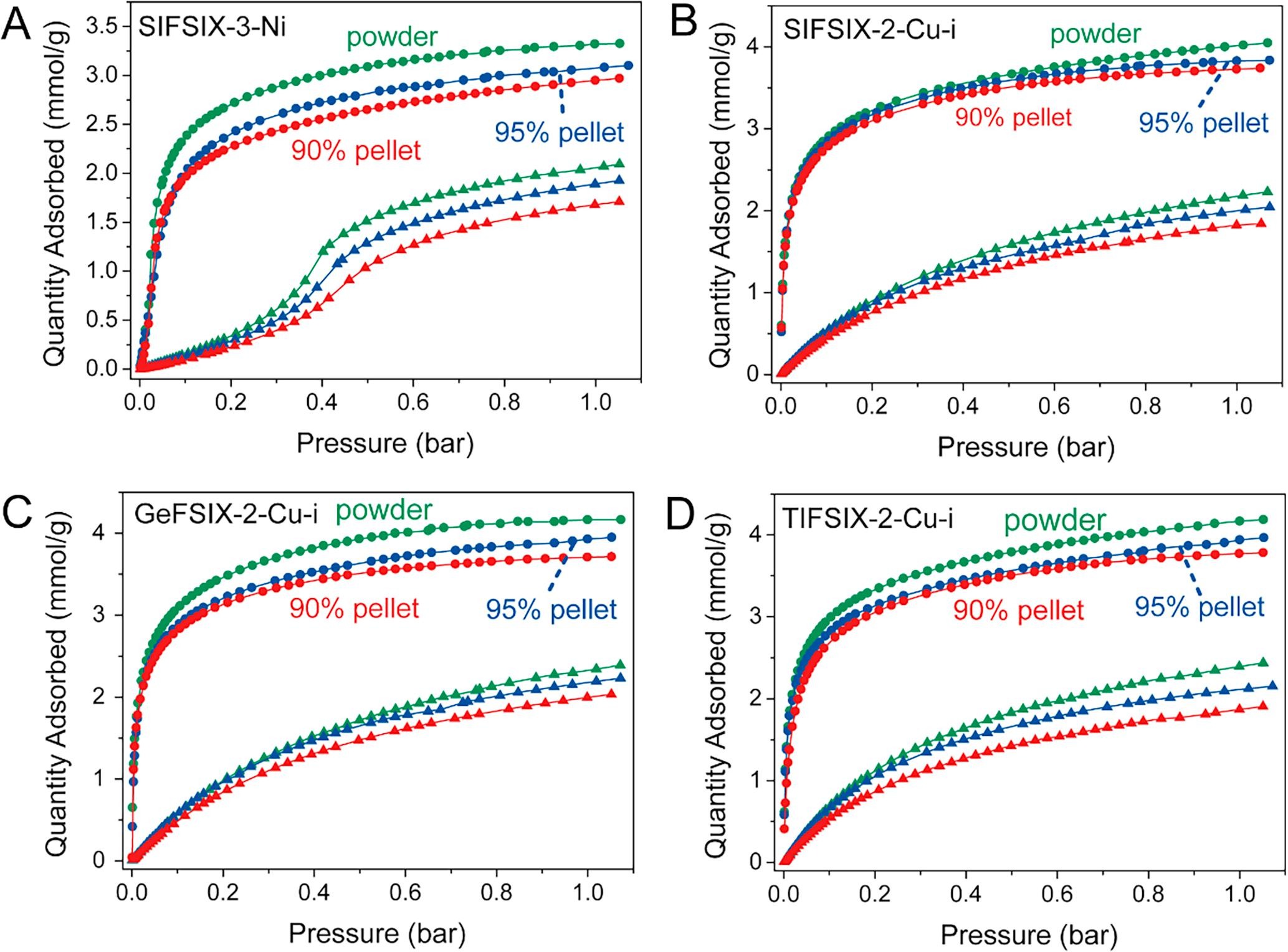
- SIFSIX-3-Ni: BET surface area decreased from 360 m²/g (powder) to 297 m²/g (90% loading pellet), pore size centered at 3–6 Å.
- SIFSIX-2-Cu-i: BET reduced by 15.2% (from 808 to 685 m²/g for 90% loading).
- GeFSIX-2-Cu-i: BET reduced by 12.7% (from 755 to 659 m²/g for 90% loading).
- TIFSIX-2-Cu-i: BET reduced by 2.9% (from 740 to 719 m²/g for 90% loading).
2.SEM tests: SEM images (e.g., GeFSIX-2-Cu-i) showed MOF crystals tightly bound by PVB, with no obvious particle size change; pellets had a cubic shape (1–2 mm) or spherical shape (4–5 mm).
3.Other tests:
- XRD: Pellets retained the crystal structure of parent MOFs, and were stable after 7 days of exposure to 70% humidity.
- TGA: Decomposition temperature of pellets (≈250 °C) was consistent with MOF powders, indicating unchanged thermal stability.
- Isosteric heat (Qst): Qst of C₂H₂ for pellets (25.9–50.6 kJ/mol) was twice that of C₂H₄, confirming stronger C₂H₂ adsorption.
3. Application
-C₂H₂/C₂H₄ separation:
- Static adsorption (298 K, 1 bar): 90% loading pellets had C₂H₂ uptake loss of 0.56%–0.93% (per gram pure MOF) but C₂H₄ loss of 5.0%–12.5%; GeFSIX-2-Cu-i pellet’s IAST selectivity (116.4) was 35% higher than powder (86.3).
- Breakthrough tests (SIFSIX-3-Ni 90% pellet, 1/99 C₂H₂/C₂H₄): C₂H₂ breakthrough time (54–66 min) was much longer than C₂H₄ (<25 min); dynamic selectivity remained ~9–10 after 3 cycles, with C₂H₄ purity >99.99%.
-SO₂ adsorption (298 K, 2000 ppm): GeFSIX-2-Cu-i pellets had 2.25 mmol/g (95% loading) and 1.96 mmol/g (90% loading) SO₂ uptake; SIFSIX-3-Ni pellets had 1.17 mmol/g (95% loading) and 1.03 mmol/g (90% loading).
-CO₂ capture (298 K, 1 bar): Mg-MOF-74 pellets had 6.01 mmol/g (95% loading) and 5.71 mmol/g (90% loading) CO₂ uptake; HKUST-1 pellets had 4.85 mmol/g (95% loading) and 4.46 mmol/g (90% loading).
4. Mechanism
-Binder-MOF compatibility: PVB’s alcoholic hydroxyl groups form hydrogen bonds with MOFs, ensuring strong adhesion and low pore blocking; its hydrophobicity and decomposition temperature (200–260 °C) match MOF degassing requirements.
-Adsorption selectivity enhancement: C₂H₂ has stronger interactions (C-H···F H-bonding, electrostatic forces) with anion-pillared MOFs than C₂H₄; PVB slightly blocks pores, more significantly reducing weak C₂H₄ adsorption (weaker binding) than strong C₂H₂ adsorption, thus enhancing selectivity.
-SO₂ adsorption mechanism: GeF₆²⁻/SiF₆²⁻ pillars in MOFs form Sδ⁺···Fδ⁻ electrostatic interactions with SO₂, enabling high low-pressure SO₂ uptake; PVB does not adsorb SO₂, so pellets retain high SO₂ capacity.
Outlook:
This research realizes the first pelletization of anion-pillared ultramicroporous MOFs with ultrahigh loading (up to 95%) using PVB, solving the contradiction between MOF loading and adsorption performance in traditional pelletization. The pellets excel in C₂H₂/C₂H₄ separation, SO₂ adsorption, and CO₂ capture, and the method is applicable to diverse MOFs. It accelerates MOF industrialization, providing efficient solutions for energy-saving gas separation and environmental protection.
Shaping of ultrahigh-loading MOF pellet with a strongly anti-tearing binder for gas separation and storage
Authors: Jieyi Zheng, Xili Cui, Qiwei Yang, Qilong Ren, Yiwen Yang, Huabin Xing
DOI: 10.1016/j.cej.2018.08.119
Link: https://www.sciencedirect.com/science/article/pii/S1385894718315936
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

