Home >
News > A Single-Molecule Propyne Trap: Highly Efficient Removal of Propyne from Propylene with Anion-Pillared Ultramicroporous Materials
A Single-Molecule Propyne Trap: Highly Efficient Removal of Propyne from Propylene with Anion-Pillared Ultramicroporous Materials
Summary:
The authors from Zhejiang University (China), National Institute of Standards and Technology (USA), and University of Texas at San Antonio (USA) developed anion-pillared ultramicroporous materials (including SIFSIX-1-Cu, SIFSIX-2-Cu-i, SIFSIX-3-Ni, etc.) with tunable pore shape, precise functional site disposition, and high adsorption selectivity. These materials achieved record-breaking results in the application field of trace propyne (C₃H₄) removal from propylene (C₃H₆) to produce polymer-grade C₃H₆.
Background:
1. To address the critical problem of deep removal of trace C₃H₄ from C₃H₆ (required to reduce C₃H₄ content to below 5 ppm or 1 ppm for polymer-grade C₃H₆, while C₃H₄/C₃H₆ have similar structures, similar molecular sizes, and ultralow C₃H₄ concentration), previous researchers mainly used energy-intensive noble metal-catalyzed hydrogenation technology for over half a century. Although this method is industrialized, it is energy-inefficient, and existing separation materials (e.g., ELM-12, [Cu(dhbc)₂(4,4'-bipy)]) have low C₃H₄ uptake under ultralow concentration (e.g., ELM-12 only has ~0.19 mmol g⁻¹ at ~700 ppm C₃H₄) and insufficient selectivity.
2. The authors in this study proposed an innovative method of constructing hybrid ultramicroporous materials with pillared inorganic anions (SiF₆²⁻, NbOF₅²⁻). They obtained materials with single-molecule trap behavior for C₃H₄, realizing ultrahigh C₃H₄ uptake under ultralow concentration (2.65 mmol g⁻¹ at 3000 ppm for SIFSIX-3-Ni) and record selectivity over C₃H₆ (>250 at 298 K).
Research Content:
1. Synthesis
The authors synthesized a series of anion-pillared ultramicroporous materials using coordination polymerization. These materials are 3D Cu/Ni/Zn coordination networks, where 2D nets composed of metal nodes (Cu, Ni, Zn) and organic linkers (4,4'-bipyridine, 4,4-dipyridylacetylene, pyrazine) are bridged by inorganic anion pillars (SiF₆²⁻, NbOF₅²⁻). Specific synthesis references include previous works (refs. [1], [27], [28]), and activation was performed before use (e.g., SIFSIX-1-Cu and SIFSIX-2-Cu-i evacuated at room temperature to pressure <7 µm Hg; SIFSIX-3-Ni, SIFSIX-3-Zn, NbOFFIVE-1-Ni degassed at 100 °C for 15 h to pressure <6 µm Hg).
2. Characterizations
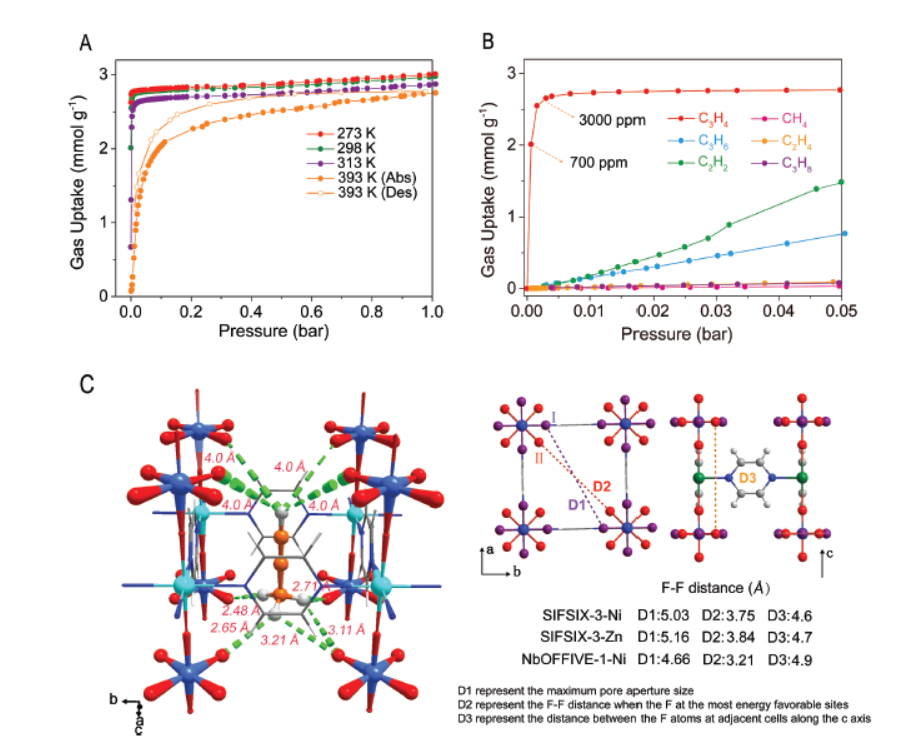
1)BET and pore size distribution: The materials are ultramicroporous. For example, SIFSIX-3-Ni has a pore cavity size of Φ5.03×7.51 Å, SIFSIX-3-Zn has Φ5.16×7.61 Å, and NbOFFIVE-1-Ni has a relatively long and thin pore cavity (4.66×7.88 Å). SIFSIX-1-Cu has a larger pore volume, enabling high saturated C₃H₄ adsorption capacity (8.76 mmol g⁻¹ at 1.0 bar, 298 K).
2)SEM/TEM tests: The article does not explicitly mention SEM/TEM test results for particle size.
3)Other tests:
-Gas adsorption isotherms (measured by ASAP 2050 Analyzer at 273–313 K): SIFSIX-3-Ni shows steep C₃H₄ adsorption at low pressure (near-saturation at 0.003 bar), with 2.0 mmol g⁻¹ uptake at ~700 ppm C₃H₄; SIFSIX-1-Cu has 8.76 mmol g⁻¹ C₃H₄ uptake at 1.0 bar, 298 K, and 10.15 mmol g⁻¹ at 273 K.
-X-ray diffraction (PXRD): Validated the single-molecule C₃H₄ trap behavior of SIFSIX-3-Ni (one C₃H₄ per unit cell) and revealed the binding mode (multiple C-Hδ⁺...Fδ⁻ hydrogen bonds with SiF₆²⁻ anions).
-Isosteric heat of adsorption (Qst) calculation: Qst of C₃H₄ on SIFSIX-3-Ni is 68 kJ mol⁻¹, indicating strong gas-physisorbent interactions.
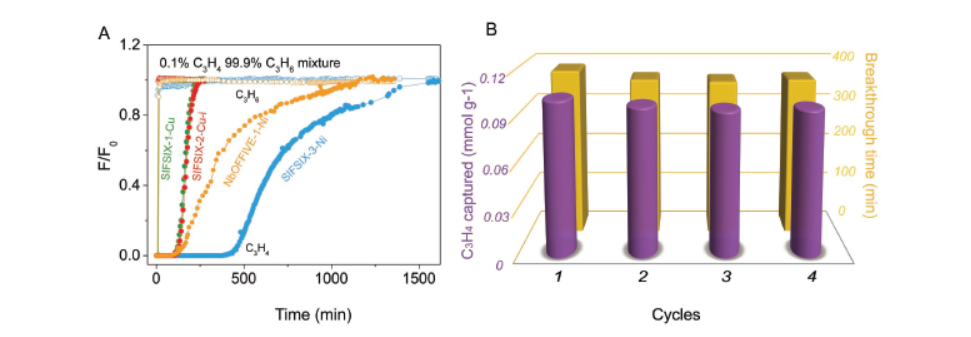
-Breakthrough experiments: Conducted with C₃H₄/C₃H₆ mixtures (0.1/99.9, 1/99 v/v) at 298 K and atmospheric pressure, showing SIFSIX-3-Ni retains C₃H₄ for 380 min (1187 mL g⁻¹) and produces C₃H₄ <2 ppm in outlet for 385 min.
3. Application
The materials were tested in the application of trace C₃H₄ removal from C₃H₆ to prepare polymer-grade C₃H₆:
- For ultralow concentration C₃H₄ (1000 ppm, 0.1/99.9 C₃H₄/C₃H₆), SIFSIX-3-Ni is the best material, with purification capacity of 1187 mL g⁻¹ and outlet C₃H₄ <2 ppm for 385 min.
- For moderate concentration C₃H₄ (10000 ppm, 1/99 C₃H₄/C₃H₆), SIFSIX-2-Cu-i is suitable, with breakthrough time of 990 min g⁻¹ (1089 mL g⁻¹), 9 times higher than the previous benchmark ELM-12 (121 mL g⁻¹).
- The materials also have potential in separating C₃ mixtures containing propane (C₃H₈), as SIFSIX-3-Ni has negligible C₃H₈ uptake at low pressure and only 1.85 mmol g⁻¹ at 1 bar (far lower than C₃H₄ and C₃H₆).
4. Mechanism
-Experimental result analysis: PXRD shows SIFSIX-3-Ni’s pore space (Φ5.03×7.51 Å) and SiF₆²⁻ anion disposition perfectly match C₃H₄’s molecular shape (4.16×4.01×6.51 ų) and interaction sites. C₃H₄ forms multiple C-Hδ⁺...Fδ⁻ hydrogen bonds (C≡Hδ⁺...Fδ⁻ ~4.0 Å, methyl C-Hδ⁺...Fδ⁻ 2.48–3.21 Å) with SiF₆²⁻ and van der Waals interactions with organic linkers, achieving one C₃H₄ per unit cell.
-Calculation and mechanism reasoning:
-DFT-D calculations: The static adsorption energy (△E) of C₃H₄ on SIFSIX-3-Ni is 82.9 kJ mol⁻¹ (much higher than 56.3 kJ mol⁻¹ for C₃H₆), explaining high selectivity; SIFSIX-1-Cu has increasing △E with C₃H₄ loading (58.4–62.1 kJ mol⁻¹) due to gas-gas interactions forming "C₃H₄ clusters"; SIFSIX-2-Cu-i has △E of 60.4 kJ mol⁻¹ for C₃H₄ (higher than 44 kJ mol⁻¹ for C₃H₆).
-IAST calculations: SIFSIX-3-Ni and NbOFFIVE-1-Ni have IAST selectivities of 290 and 360 for C₃H₄/C₃H₆ (1/999 to 1/10 molar ratio), far higher than ELM-12 (89).
Outlook:
This research innovatively developed anion-pillared ultramicroporous materials with single-molecule trap function for C₃H₄, breaking the bottleneck of low uptake and poor selectivity of traditional materials for trace C₃H₄ separation. It provides an energy-efficient alternative to industrial noble metal hydrogenation technology, setting new benchmarks for trace C₃H₄ removal from C₃H₆. Moreover, the strategy of precisely matching pore size/shape and functional sites with guest molecules is instructive for designing high-performance materials for other gas separations (e.g., CO₂ capture, SO₂ removal).
A Single-Molecule Propyne Trap: Highly Efficient Removal of Propyne from Propylene with Anion-Pillared Ultramicroporous Materials
Authors: Lifeng Yang, Xili Cui, Qiwei Yang, Siheng Qian, Hui Wu, Zongbi Bao, Zhiguo Zhang, Qilong Ren, Wei Zhou, Banglin Chen, Huabin Xing*
DOI: 10.1002/adma.201705374
Link: https://onlinelibrary.wiley.com/doi/10.1002/adma.201705374
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

