Home >
News > Direct covalent post-synthetic chemical modification of Cr-MIL-101 using nitrating acid
Direct covalent post-synthetic chemical modification of Cr-MIL-101 using nitrating acid
Summary:
The authors from Christian-Albrechts-Universität zu Kiel and Institut Lavoisier de Versailles developed a post-synthetically modified Cr-MIL-101 MOF with covalently grafted nitro, amino, and urea functionalities, achieving enhanced chemical versatility and porosity for advanced functional materials applications.
Background:
1. To address the challenge of functionalizing thermally robust MOFs, previous researchers attempted direct synthesis of amino-functionalized analogs, but high-temperature hydrothermal conditions often decompose sensitive linkers, limiting functional group incorporation.
2. The authors proposed an innovative post-synthetic modification strategy using nitrating acid to covalently introduce –NO₂ groups into the Cr-MIL-101 framework, followed by reduction to –NH₂ and further urea formation, successfully circumventing thermal instability issues.
Research Content:
1. Synthesis:
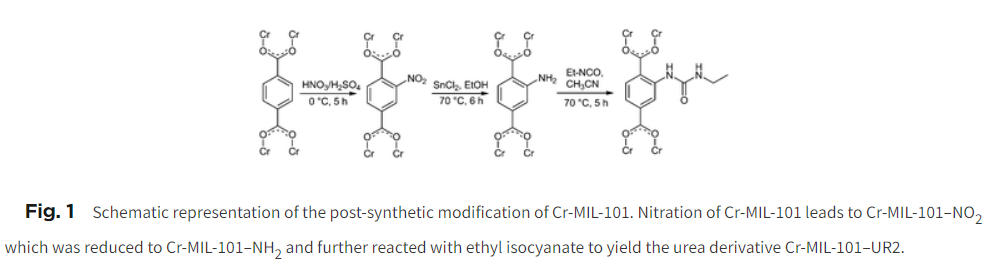
Cr-MIL-101 was nitrated using concentrated HNO₃/H₂SO₄ at 0 °C to yield Cr-MIL-101–NO₂, or directly synthesized via hydrothermal reaction with nitroterephthalic acid. Subsequent reduction with SnCl₂/EtOH produced Cr-MIL-101–NH₂, which was reacted with ethyl isocyanate to form the urea derivative Cr-MIL-101–UR2.
2. Characterizations:
1) BET surface area decreased from 2600 m²/g (Cr-MIL-101) to 1425 m²/g (Cr-MIL-101–NO₂), and increased to ~2300 m²/g after activation of Cr-MIL-101–NH₂.
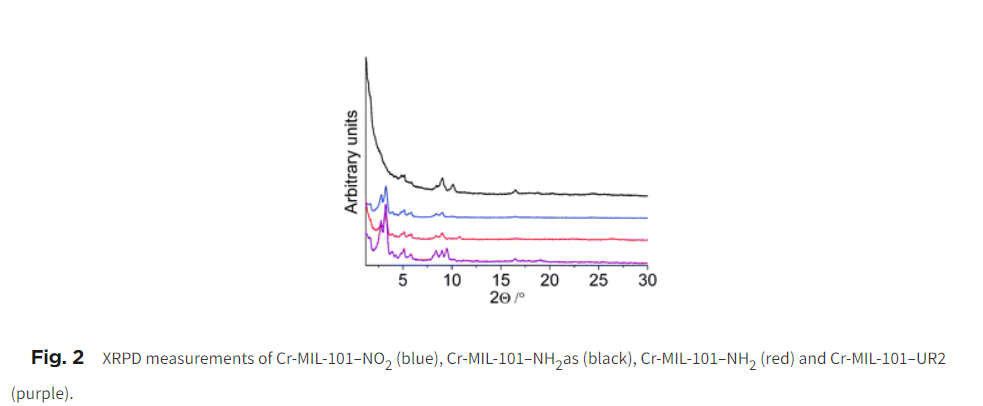
2) XRPD confirmed retention of crystallinity and framework integrity throughout modifications.
3) IR and NMR spectroscopy confirmed successful covalent grafting of –NO₂, –NH₂, and urea groups; TGA showed high thermal stability.
3. Application:
While no direct application testing was reported, the urea-functionalized MOF (Cr-MIL-101–UR2) demonstrates potential for selective adsorption, catalysis, or drug delivery due to its high porosity and tunable surface chemistry.
4. Mechanism:
The nitration proceeds via electrophilic aromatic substitution on the terephthalate linker; the nitro group deactivates the ring, preventing over-nitration. Reduction to –NH₂ enables further urea formation via isocyanate addition, validating a robust post-synthetic functionalization pathway.
Outlook:
This work establishes a powerful post-synthetic toolkit for functionalizing thermally stable MOFs like Cr-MIL-101, expanding their chemical diversity without compromising structural integrity. The strategy opens new avenues for designing task-specific MOFs for catalysis, sensing, and separation.
Direct covalent post-synthetic chemical modification of Cr-MIL-101 using nitrating acid
Authors: Stephan Bernt, Vincent Guillerm, Christian Serre, Norbert Stock
DOI: 10.1039/c0cc04526h
Link: https://pubs.rsc.org/en/content/articlelanding/2011/cc/c0cc04526h
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

