Home >
News > Mechanochemical synthesis of a robust cobalt-based metal–organic framework for adsorption separation methane from nitrogen
Mechanochemical synthesis of a robust cobalt-based metal–organic framework for adsorption separation methane from nitrogen
Summary:
The authors from multiple institutions (including Nanjing Tech University, Guangdong University of Technology, and others) developed a robust cobalt-based metal–organic framework (Co(AIP)(BPY)₀.₅) via mechanochemical synthesis. The material demonstrated high methane (CH₄) uptake (1.03 mmol/g) and CH₄/N₂ selectivity (7.3), showing promising performance in gas separation applications.
Background:
1. To address the challenge of separating CH₄ from N₂—due to their similar physical properties—previous researchers explored porous adsorbents like zeolites and carbon materials, achieving limited success due to low selectivity and uptake.
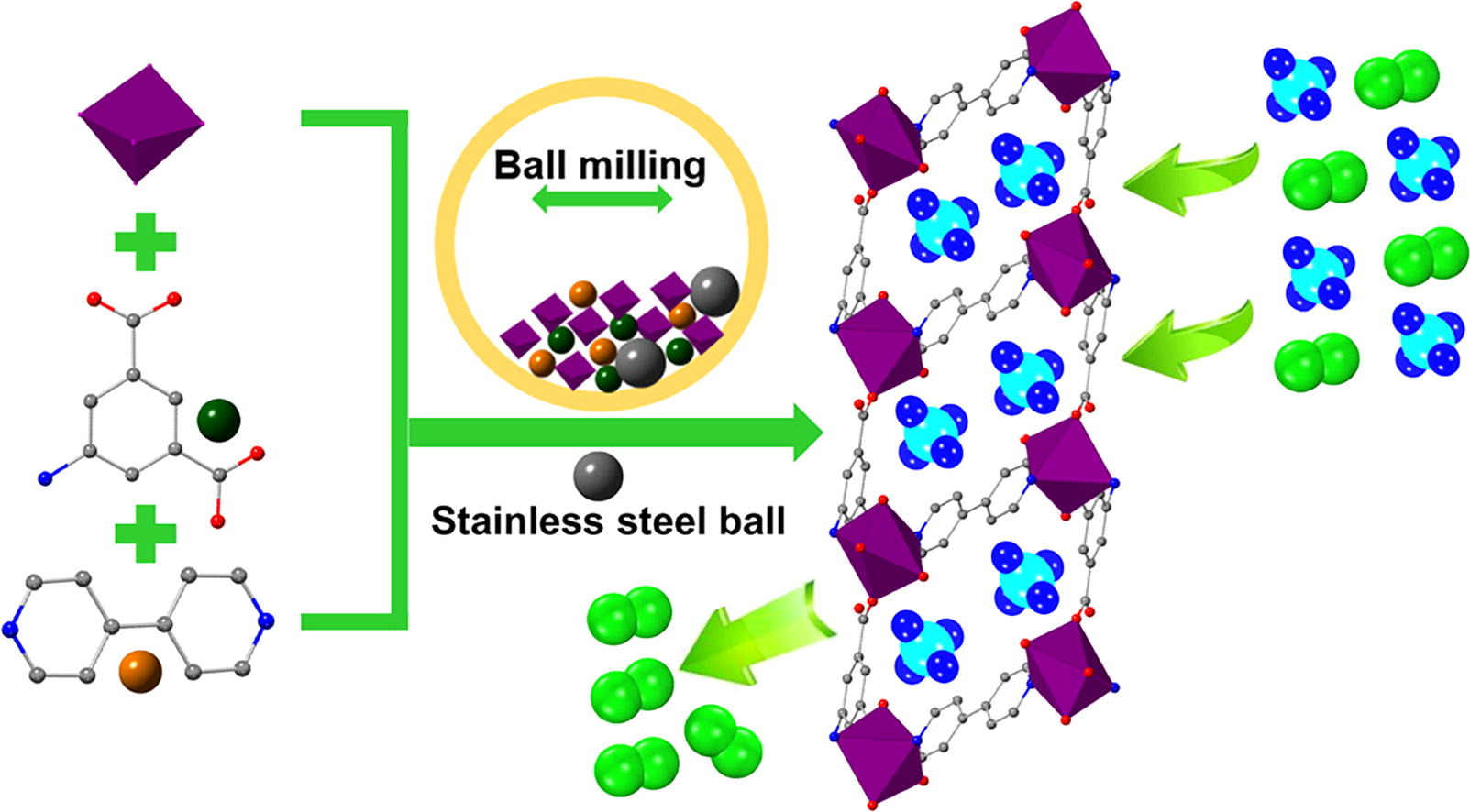
2. The authors proposed a novel, solvent-free mechanochemical synthesis method to produce a water-stable, CH₄-selective MOF, achieving gram-scale production and competitive separation performance.
Research Content:
1. Synthesis:
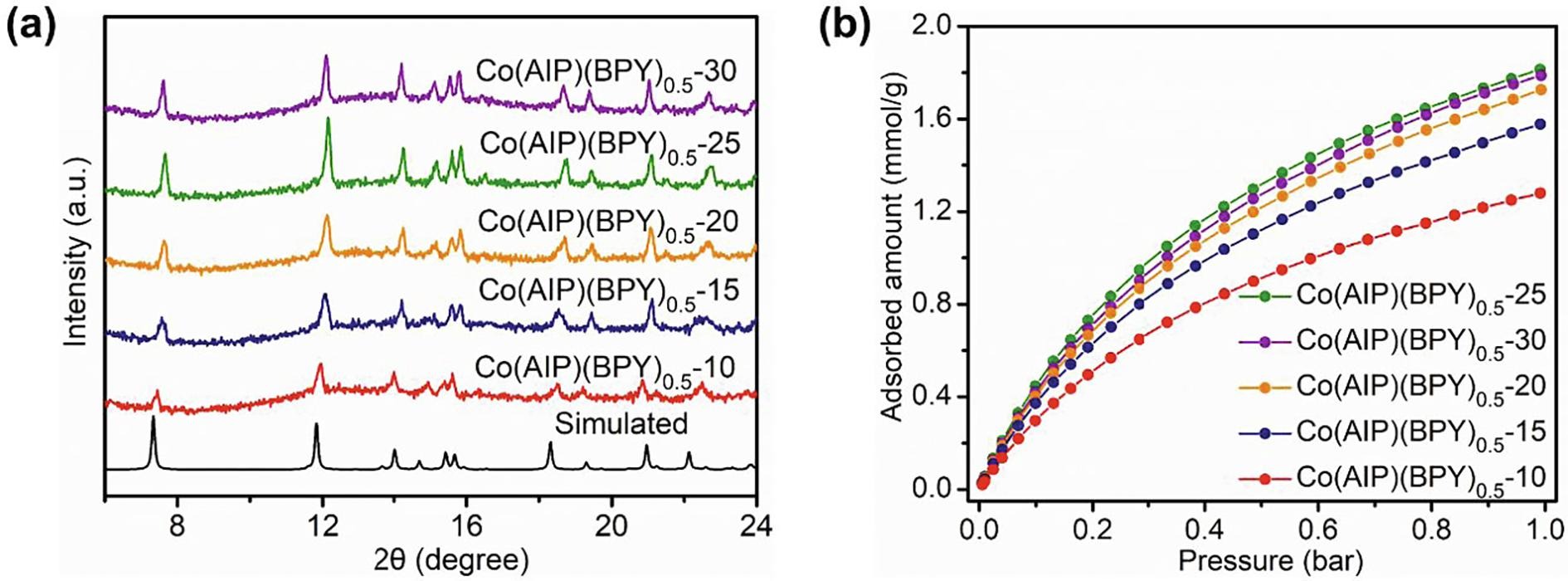
The authors synthesized Co(AIP)(BPY)₀.₅ using a mechanochemical ball-milling method without solvents. The grinding time was optimized to 25 minutes to achieve the best porosity and crystallinity.
2. Characterizations:
- CO₂ adsorption isotherms indicated high porosity in the optimized sample (Co(AIP)(BPY)₀.₅–25).
- SEM images showed irregular plate-like morphology with improved surface smoothness at longer grinding times.
- PXRD and FTIR confirmed successful MOF formation and structural integrity.
- TGA showed thermal stability up to 250 °C.
3. Application:
The material was tested for CH₄/N₂ separation. At 298 K and 5 bar, CH₄ uptake was 1.03 mmol/g, while N₂ uptake was only 0.26 mmol/g, yielding a selectivity of 7.3. Simulated breakthrough curves confirmed efficient separation under various gas compositions.
4. Mechanism:
Molecular simulations revealed that CH₄ molecules (spheroidal) preferentially adsorb in the pore centers, while N₂ molecules (linear) are located near pore walls. This difference in binding energy (CH₄: −11.33 kJ/mol; N₂: −2.91 kJ/mol) underpins the selective adsorption behavior.
Outlook:
This work demonstrates a scalable, eco-friendly route to synthesize a water-stable MOF with competitive CH₄/N₂ separation performance. The material’s stability, selectivity, and moderate uptake make it a promising candidate for industrial gas purification applications.
Mechanochemical synthesis of a robust cobalt-based metal–organic framework for adsorption separation methane from nitrogen
Authors: Chenghui Zhang, Yongwei Chen, Houxiao Wu, Huilin Li, Xinyuan Li, Shi Tu, Zhiwei Qiao, Dongli An, Qibin Xia
DOI: 10.1016/j.cej.2021.133876
Link: https://doi.org/10.1016/j.cej.2021.133876
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

