Home >
News > Iodine Capture Using Zr-based Metal-Organic Frameworks (Zr-MOFs): Adsorption Performance and Mechanism
Iodine Capture Using Zr-based Metal-Organic Frameworks (Zr-MOFs): Adsorption Performance and Mechanism
Summary:
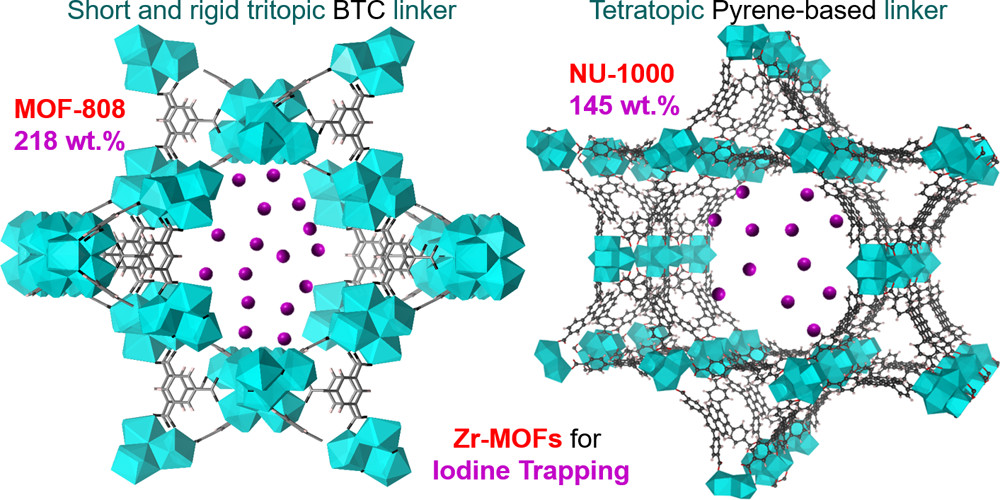
The authors from Tianjin University (China) and Xi'an University of Architecture and Technology (China) developed a series of Zr-based metal-organic frameworks (Zr-MOFs) with a Zr₆(μ₃-O)₄(μ₃-OH)₄ cluster and various carboxylate linkers, achieving excellent performance in the application of volatile radioiodine capture.
Background:
1. To address the problem of efficient and stable capture of radioactive iodine (e.g., ¹²⁹I with a 15.7-million-year half-life) from nuclear-related activities, previous researchers used traditional adsorbents (activated charcoal, silver-containing zeolites) and some MOFs (ZIF-8, Cu-BTC, MFM-300) for iodine capture, achieving certain adsorption capacities, yet there are problems such as low structural stability of MOFs, inefficient recovery of traditional adsorbents, and unclear iodine capture mechanism of Zr-MOFs.
2. The authors in this study proposed an innovative method of systematically investigating 9 Zr-MOFs (5 original, 4 imidazole/pyridine-modified via ligand exchange) and combining multiple characterizations (PXRD, Raman, XPS) with DFT calculations, obtaining results that clarify the relationship between Zr-MOF structure and iodine capture performance and reveal the adsorption mechanism.
Research Content:
1. Synthesis
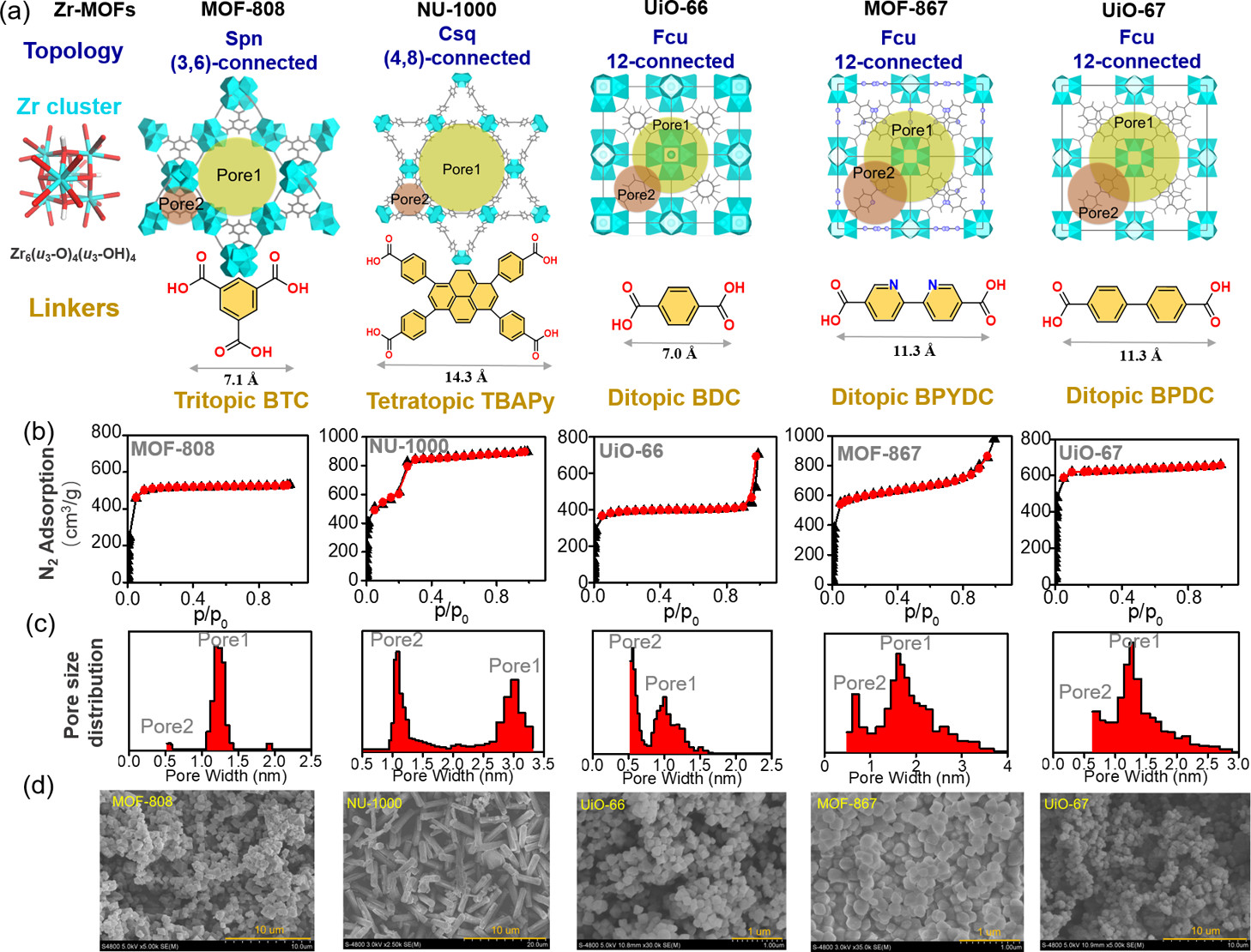
The authors synthesized 5 original Zr-MOFs (MOF-808, NU-1000, MOF-867, UiO-66, UiO-67) via hydrothermal method: MOF-808 was synthesized by dissolving H₃BTC and ZrOCl₂·8H₂O in DMF/formic acid, heating at 130 °C for 48 h; NU-1000 by dissolving ZrCl₄ and benzoic acid in DMF, adding H₄TBAPy, heating at 120 °C for 48 h; UiO-66/UiO-67/MOF-867 by reacting ZrCl₄ with H₂BDC/H₂BPDC/H₂BPYDC respectively. 4 modified Zr-MOFs (MOF-808-imidazole/pyridine, NU-1000-imidazole/pyridine) were synthesized via ligand exchange of terminal ligands on Zr clusters with 2-imidazolecarboxylic acid (ImC)/pyridine-2-carboxylic acid (PyC) ligands.
2. Characterizations
1.BET and pore size distribution: BET surface areas of UiO-67, MOF-867, NU-1000, MOF-808, UiO-66 are 2638, 2403, 2126, 1930, 1072 m²/g respectively; pore volumes are 1.17, 1.12, 1.27, 0.82, 0.53 cm³/g respectively. After iodine elution, MOF-808 retains 63% pore volume (0.52 cm³/g), UiO-67 only 12% (0.14 cm³/g).
2.SEM tests: NU-1000 (hexagonal prism) has a size of 2 μm×8 μm; MOF-808/UiO-67 (octahedral) are 0.5-1 μm; MOF-867/UiO-66 are 100-200 nm.
3.Other tests: PXRD shows MOF-808 retains lattice structure (a=35.278 Å→35.253 Å) after 10% iodine loading; XPS detects I₂ (620.1, 631.7 eV) and I₃⁻ (618.6, 630.1 eV) in iodine-loaded Zr-MOFs; Raman shows peaks at 110 cm⁻¹ (I₃⁻/I₂⁻) and 170 cm⁻¹ (pore I₂) for iodine-loaded MOF-808.
3. Application
The materials were tested in volatile iodine capture: MOF-808 has the highest adsorption capacity (2.18 g/g at 80 °C); under 18% RH (saturated CaCl₂ solution), MOF-808 still reaches 1.52 g/g; after 3 cycles, MOF-808/NU-1000 retain 70% re-adsorption ratio. Modified Zr-MOFs (MOF-808-imidazole: 1.34 g/g, MOF-808-pyridine: 0.89 g/g) have lower capacities than original ones.
4. Mechanism
- Experimental analysis: High-connected/rigid linkers (e.g., MOF-808’s tritopic BTC) enhance Zr-MOF stability; initial iodine adsorption is via framework internal boundary (110 cm⁻¹ in Raman), later forming close-packed I₂ (170 cm⁻¹); FTIR confirms linkers remain coordinated with Zr clusters during adsorption-desorption.
- Calculation/mechanism: DFT (RM06L method, LANL2DZ for Zr/I, 6-31G(d,p) for others) shows terminal -OH in MOF-808 has strong affinity for I₂ (ΔE=-54 kJ/mol); N-heterocycles (imidazole/pyridine) enhance iodine affinity via charge transfer, but reduce pore volume (MOF-808-pyridine: BET 1379 m²/g, pore volume 0.49 cm³/g) and stability of Zr-MOFs.
Outlook:
This research clarifies the structure-performance relationship of Zr-MOFs for iodine capture, identifies MOF-808 as a promising radioiodine adsorbent, and provides guidance for designing MOFs with rigid/multitopic linkers and N-heterocycles for efficient adsorption.
Iodine Capture Using Zr-based Metal-Organic Frameworks (Zr-MOFs): Adsorption Performance and Mechanism
Authors: Peng Chen, Xihong He, Maobin Pang, Xiuting Dong, Song Zhao, Wen Zhang*
DOI: 10.1021/acsami.0c02129
Link: https://pubs.acs.org/doi/10.1021/acsami.0c02129
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

