Home >
News > Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene
Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene
Summary:
The authors from Zhejiang University (China), University of Limerick (Ireland), University of Amsterdam (Netherlands), National Institute of Standards and Technology (USA), King Abdullah University of Science and Technology (Saudi Arabia), and University of Texas–San Antonio (USA) developed SIFSIX-series metal-organic frameworks (MOFs) including SIFSIX-1-Cu ([Cu(4,4'-bipyridine)₂(SiF₆)]ₙ), SIFSIX-2-Cu ([Cu(dpa)₂(SiF₆)]ₙ), SIFSIX-2-Cu-i ([Cu(dpa)₂(SiF₆)]ₙ·1.7C₂D₂), SIFSIX-3-Zn ([Zn(pyr)₂(SiF₆)]ₙ), and SIFSIX-3-Ni ([Ni(pyr)₂(SiF₆)]ₙ). These materials have tunable pore chemistry/size and strong C₂H₂ binding ability, achieving exceptional performance in C₂H₂/C₂H₄ separation (trace C₂H₂ removal and binary mixture separation) in industrial polymer-grade gas production.
Background:
1. To address the need for efficient C₂H₂ capture from C₂H₄ (critical for polymer-grade C₂H₂/C₂H₄ production), previous researchers developed porous materials like MOF-74 (high C₂H₂ uptake via open metal sites but low selectivity) and M’MOF (high selectivity via ultramicropore sieving but low C₂H₂ uptake). Existing industrial methods (solvent absorption, partial hydrogenation) are also energy-intensive, lacking a balance between adsorption capacity and selectivity.
2. The authors proposed an innovative strategy: designing SIFSIX-series MOFs with preformed SiF₆²⁻ anions (pillars) and organic linkers. By tuning pore size (via linker length/framework interpenetration) and leveraging SiF₆²⁻-mediated specific interactions, these MOFs achieve a "sweet spot" of high C₂H₂ capacity and selectivity, outperforming traditional materials.
Research Content:
1. Synthesis
The authors synthesized SIFSIX-series MOFs via room-temperature diffusion (consistent with reported methods for SIFSIX materials), using metal salts and organic linkers as precursors:
- Precursor 1,2-bis(4-pyridyl)acetylene (dpa): Synthesized via bromination of trans-1,2-bis(4-pyridyl)ethylene and dehydrobromination with Na in t-BuOH (overall yield 43%, as in prior reports).
- SIFSIX-1-Cu ([Cu(4,4'-bipyridine)₂(SiF₆)]ₙ): Room-temperature diffusion of methanol solution of 4,4'-bipyridine into methanol solution of CuSiF₆, forming primitive cubic topology frameworks.
- SIFSIX-2-Cu ([Cu(dpa)₂(SiF₆)]ₙ): Room-temperature diffusion of ethanol solution of dpa into ethylene glycol solution of CuSiF₆, yielding non-interpenetrated frameworks with 10.5 Å×10.5 Å cavities.
- SIFSIX-2-Cu-i ([Cu(dpa)₂(SiF₆)]ₙ·1.7C₂D₂): Room-temperature diffusion of methanol solution of CuSiF₆ into DMSO solution of dpa, forming doubly interpenetrated frameworks (pore size reduced vs. SIFSIX-2-Cu).
- SIFSIX-3-Zn ([Zn(pyr)₂(SiF₆)]ₙ) / SIFSIX-3-Ni ([Ni(pyr)₂(SiF₆)]ₙ): Room-temperature diffusion of methanol solution of pyrazine (pyr) into methanol solution of ZnSiF₆/NiSiF₆, forming ultramicroporous frameworks.
2. Characterizations
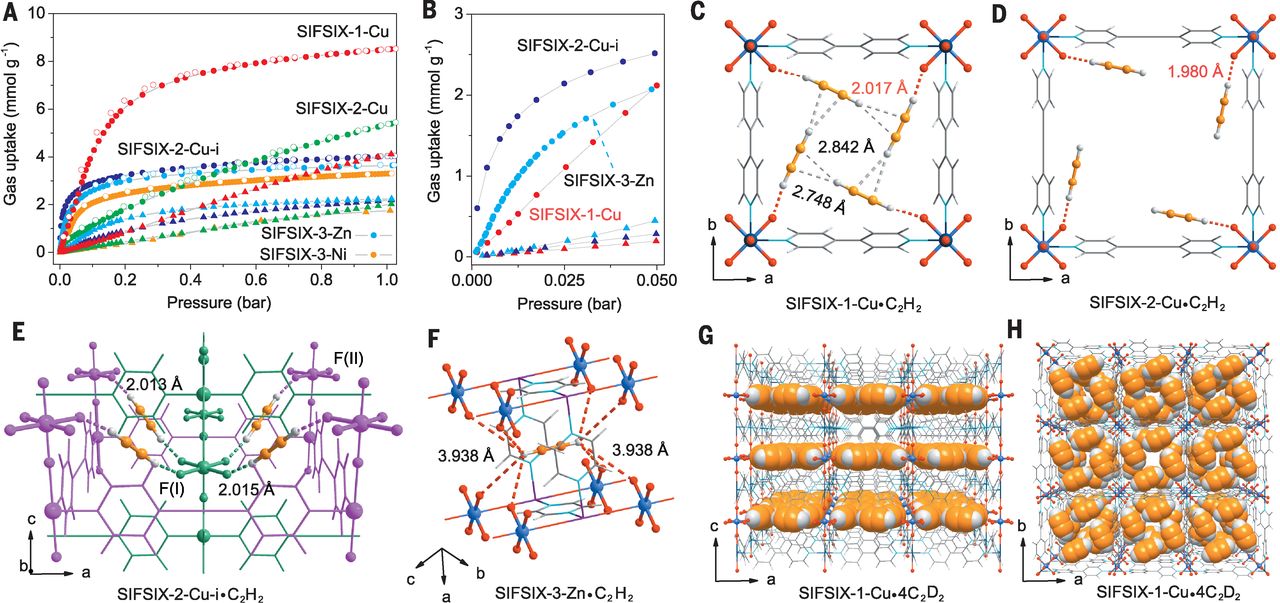
1. BET and Pore Size:
- SIFSIX-1-Cu: Large surface area (consistent with primitive cubic topology), pore size optimized for high C₂H₂ uptake (8.5 mmol/g at 298 K/1.0 bar); volumetric C₂H₂ uptake = 0.191 g/cm³ (highest among SIFSIX materials).
- SIFSIX-2-Cu: Wider pores (10.5 Å×10.5 Å), lower C₂H₂ adsorption energy (34.6 kJ/mol) and uptake (5.3 mmol/g at 298 K/1.0 bar) vs. SIFSIX-1-Cu.
- SIFSIX-2-Cu-i: Interpenetration reduces pore size, enabling high low-pressure C₂H₂ uptake (2.1 mmol/g at 298 K/0.025 bar); pore volume C₂H₂ storage density = 0.403 g/cm³.
- SIFSIX-3-Zn/SIFSIX-3-Ni: Ultramicropores (smallest among the series), C₂H₂ storage density = 0.499 g/cm³ (SIFSIX-3-Zn), but lower total uptake vs. SIFSIX-1-Cu.
2. Neutron Powder Diffraction / XRD:
- Neutron powder diffraction (200 K) for SIFSIX-1-Cu·4C₂D₂ and SIFSIX-2-Cu-i·1.7C₂D₂ confirmed ordered C₂H₂ adsorption: SIFSIX-1-Cu has 4 C₂D₂ molecules per unit cell (C-D···F H-bond length = 2.063 Å); SIFSIX-2-Cu-i has C₂H₂ bound to two SiF₆²⁻ anions (dual C-D···F H-bonds, 2.134 Å).
- Powder XRD (post-breakthrough experiments) confirmed structural stability of all SIFSIX materials after cyclic adsorption.
3. Other Tests:
- DFT-D Calculations: Quantified C₂H₂ binding energies: SIFSIX-2-Cu-i (52.9 kJ/mol) > SIFSIX-3-Zn (50.3 kJ/mol) > SIFSIX-1-Cu (44.6 kJ/mol) > SIFSIX-2-Cu (34.6 kJ/mol); C₂H₂ binding energy > C₂H₄ (e.g., 52.9 vs. 39.8 kJ/mol in SIFSIX-2-Cu-i) due to stronger C-H···F H-bonds.
- Moisture/Oxygen/CO₂ Tolerance: PXRD and breakthrough tests showed SIFSIX-2-Cu-i retains performance in presence of moisture (6–1340 ppm), O₂ (2200 ppm), and CO₂ (10–1000 ppm).
3. Application
The MOFs were tested for C₂H₂/C₂H₄ separation in industrial scenarios, with results as follows:
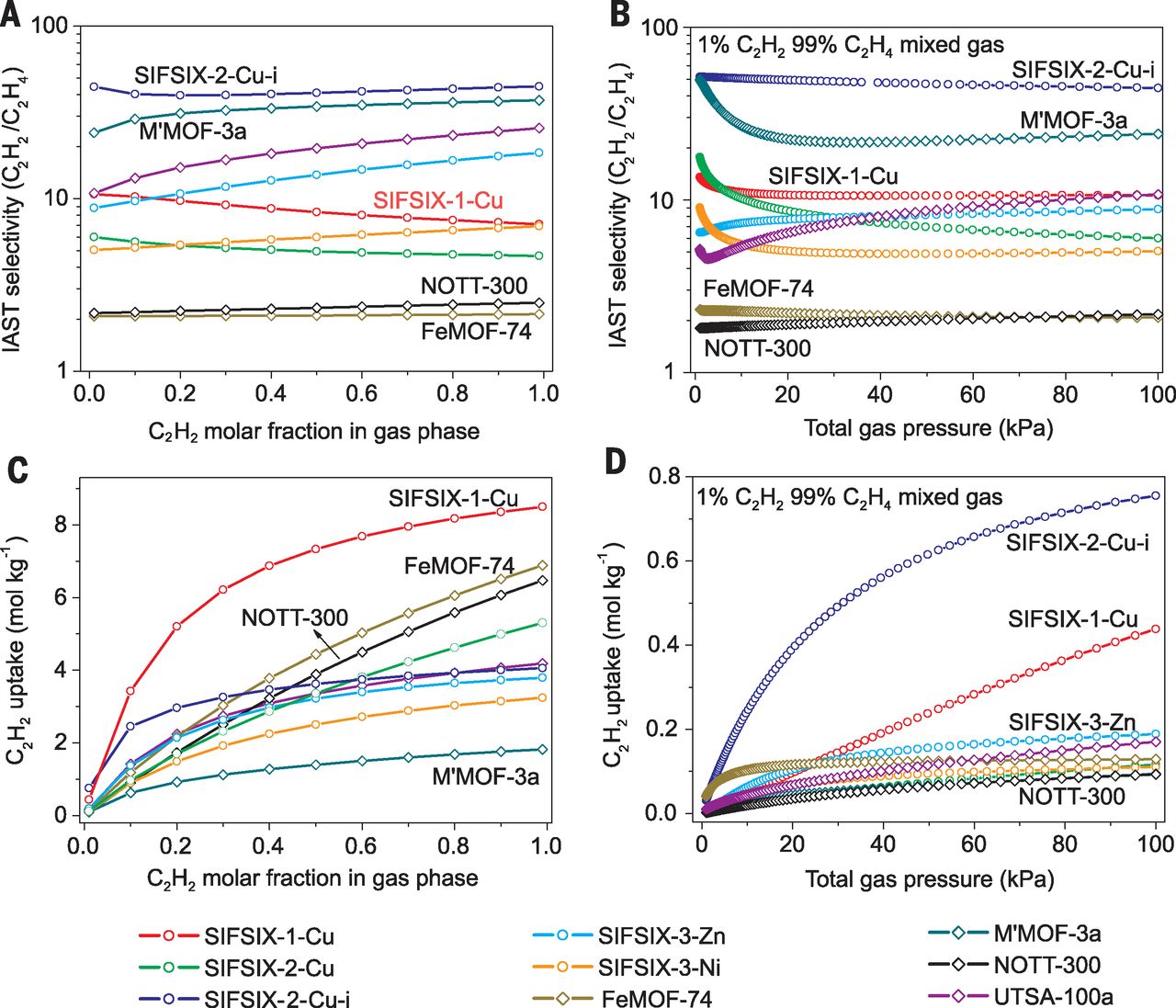
1. Trace C₂H₂ Removal (1% C₂H₂/99% C₂H₄, 298 K/1.01 bar):
- SIFSIX-2-Cu-i: Best performance—C₂H₂ uptake = 0.73 mmol/g; outlet C₂H₂ < 2 ppm for 140 min, C₂H₄ purity > 99.998%; IAST selectivity = 39.7–44.8 (record for MOFs); stable for 16 cycles.
- SIFSIX-1-Cu: C₂H₂ uptake = 0.38 mmol/g; longer breakthrough time than SIFSIX-3-Zn due to higher selectivity (10.6 vs. 8.8 for SIFSIX-3-Zn).
- SIFSIX-3-Zn: C₂H₂ uptake = 0.08 mmol/g; lower performance vs. SIFSIX-2-Cu-i/1-Cu.
2. Binary Mixture Separation (50% C₂H₂/50% C₂H₄, 298 K/1.01 bar):
- SIFSIX-1-Cu: Best capacity—C₂H₂ uptake = 6.37 mmol/g (37% higher than FeMOF-74); IAST selectivity = 7.1–10.6 (higher than FeMOF-74: 2.1, NOTT-300: 2.2–2.5).
- SIFSIX-2-Cu-i: C₂H₂ uptake = 2.88 mmol/g; lower capacity vs. SIFSIX-1-Cu but higher selectivity.
- SIFSIX-3-Zn: C₂H₂ uptake = 1.52 mmol/g; limited by ultramicropore size.
3. Cyclability: SIFSIX-2-Cu-i (16 cycles) and SIFSIX-3-Zn (3 cycles) showed no performance decline; all MOFs retained crystallinity post-tests.
4. Mechanism
- Specific Binding: Weakly basic SiF₆²⁻ anions (pKa = 1.92) form strong C-H···F H-bonds with weakly acidic C₂H₂ (pKa = 25) vs. less acidic C₂H₄ (pKa = 44). This gives higher C₂H₂ binding energy (e.g., 52.9 vs. 39.8 kJ/mol in SIFSIX-2-Cu-i).
- Guest-Guest Synergy: SIFSIX-1-Cu has optimal spacing between adsorbed C₂H₂ molecules, enabling Hᵟ⁺···Cᵟ⁻ dipole-dipole interactions (3.063–3.128 Å), further enhancing adsorption energy (44.6 → 47.0 kJ/mol).
- Pore Size Effect: Interpenetration (SIFSIX-2-Cu-i) reduces pore size for low-pressure C₂H₂ capture; SIFSIX-1-Cu’s larger pores enable high total uptake; SIFSIX-3-Zn’s ultramicropores favor selectivity but limit capacity.
Outlook:
This research achieves a breakthrough in balancing C₂H₂ adsorption capacity and selectivity via SIFSIX-series MOFs. By tuning pore chemistry (SiF₆²⁻ pillars) and size (linker/interpenetration), the materials outperform traditional adsorbents in industrial C₂H₂/C₂H₄ separation—SIFSIX-2-Cu-i for trace C₂H₂ removal and SIFSIX-1-Cu for binary mixture separation. The design principle (specific binding sites + synergistic guest interactions) provides a modular strategy for developing porous materials for other gas separations (e.g., CO₂, H₂), addressing energy-intensive industrial gas purification challenges.
Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene
Authors: Xili Cui, Kaijie Chen, Huabin Xing, Qiwei Yang, Rajamani Krishna, Zongbi Bao, Hui Wu, Wei Zhou, Xinglong Dong, Yu Han, Bin Li, Qilong Ren, Michael J. Zaworotko, Banglin Chen
DOI: 10.1126/science.aaf2458
Link: https://www.science.org/doi/10.1126/science.aaf2458
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

