Product: Cu(QcQc)
Synonyms: Cu(Qc)2 ; [Cu(quinoline‐5‐carboxyate)2] ; Qc‐5‐Cu-sql
CAS:2381226-20-4
Basic Information
| Unit MF. | C20H12N2O4Cu | Unit MW. | 407.87 | ||
| Coordination Metal | Cu | Linkers |
Quinoline-5-carboxylic acid (CAS:7250-53-5) |
||
| Pore Size | 0.33-0.38nm | Pore volume | |||
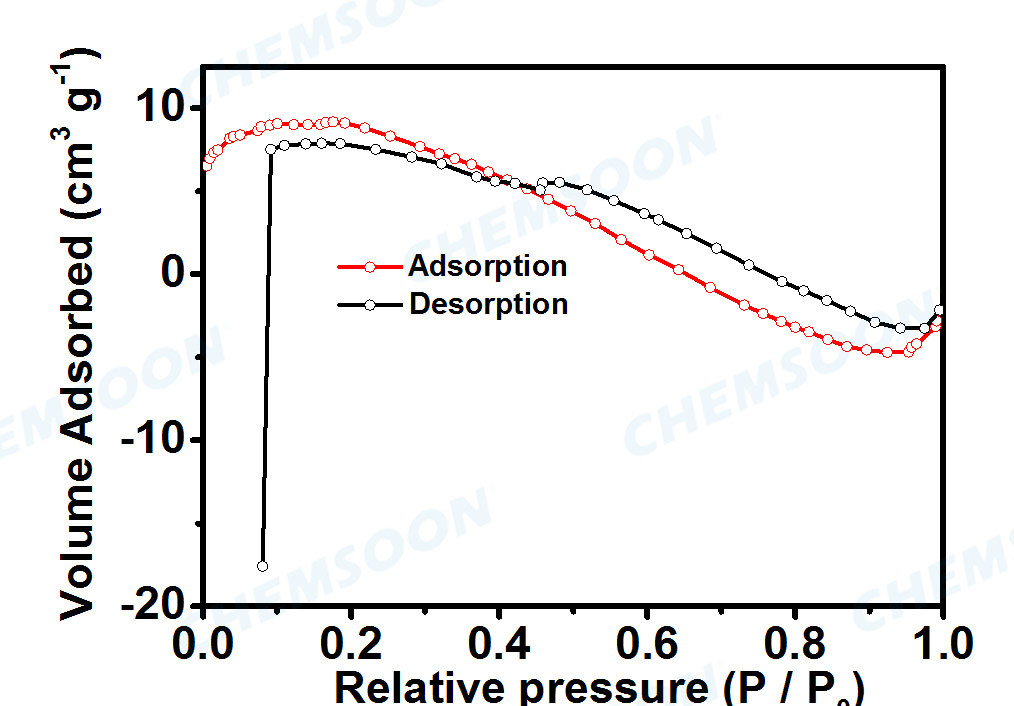
| Surface Area | No N2 adsorption | ||||
| Analog Structure | 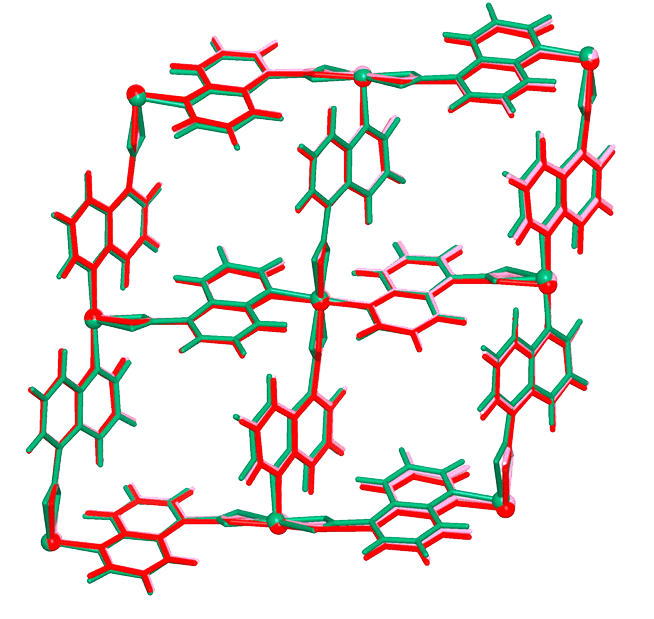 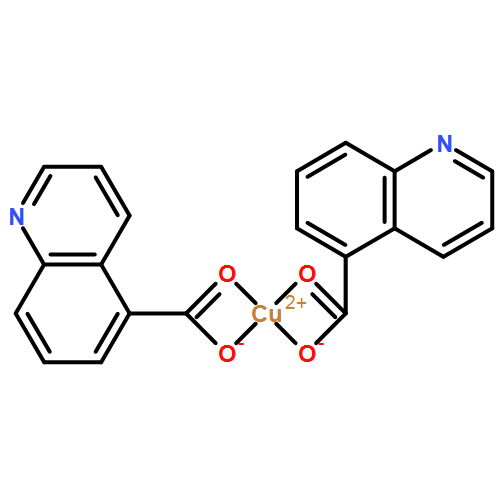 |
||||
Product Property
| Appearance | Blue Powder | |||
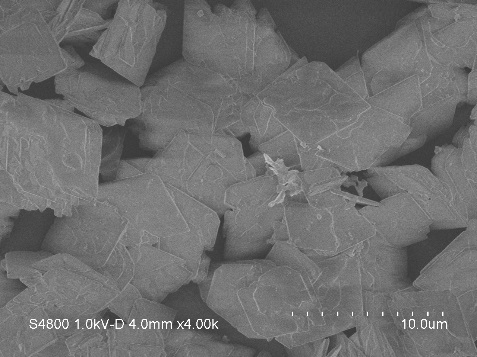
| Particle Size | 5-10um irregular bulk or rhombus nanoparticles | |||
Stability
1) Cu(Qc)2 is stable in air and aqueous conditions for months
2) Thermal decomposition temperature around 240 ° C
2) Thermal decomposition temperature around 240 ° C
Preservation
1) Keep sealed in dry and cool condition
2) It is recommended to activate for 12 hours at 120 degree in vacuum
Other Features
Fluorescence:NA
Applications
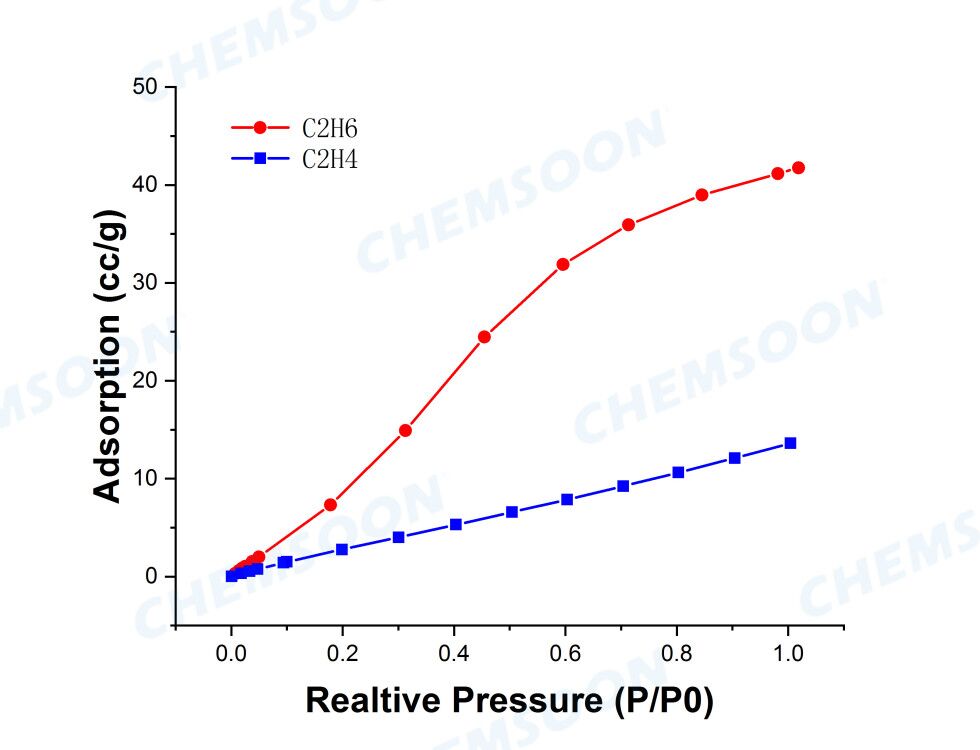
1) Gas (e.g. carbon dioxide, C2H4/C2H6) sorption and separation
2) vertical pore tunnel for transportion of ions
2) vertical pore tunnel for transportion of ions
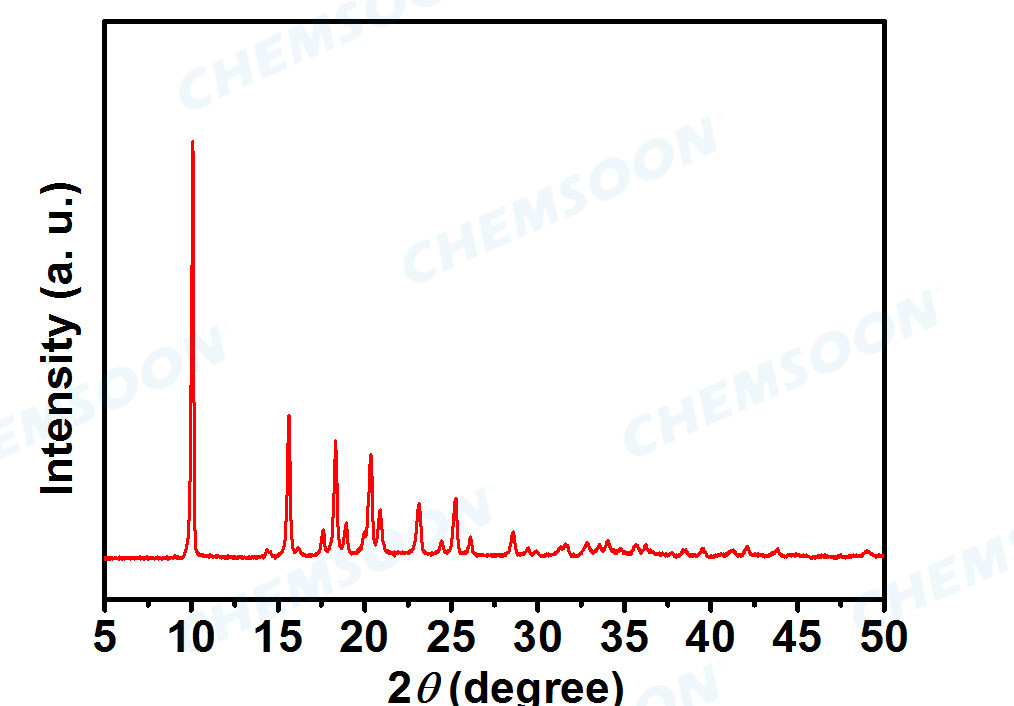
Characterizations
References
1) Chen, Kai-Jie; Madden, David G.; Pham, Tony; Forrest, Katherine A.; Kumar, Amrit; Yang, Qing-Yuan; Xue, Wei; Space, Brian; Perry, John J. IV; Zhang, Jie-Peng; Chen, Xiao-Ming; Zaworotko, Michael J.; Angew. Chem., Int. Ed. 2016, 55(35), 10268-10272, DOI: 10.1002/anie.201603934 ; Tuning Pore Size in Square‐Lattice Coordination Networks for Size‐Selective Sieving of CO2
2)Lin, Rui-Biao; Wu, Hui; Li, Libo; Tang, Xiao-Liang; Li, Zhiqiang; Gao, Junkuo; Cui, Hui; Zhou, Wei; Chen, Banglin; J. Am. Chem. Soc. 2018, 140, 40, 12940-12946, DOI: 10.1021/jacs.8b07563 ; Boosting Ethane/Ethylene Separation within Isoreticular Ultramicroporous Metal–Organic Frameworks
3) Tang, Yuning; Wang, Sa; Zhou, Xin; Wu, Ying; Xian, Shikai; Li, Zhong; Chem. Eng. Sci. 2020, 213, 115355, DOI: 10.1016/j.ces.2019.115355 ; Room temperature synthesis of Cu(Qc)2 and its application for ethane capture from light hydrocarbons