Home >
News > Organic–Inorganic Hybrid Polymer-Encapsulated Magnetic Nanobead Catalysts
Organic–Inorganic Hybrid Polymer-Encapsulated Magnetic Nanobead Catalysts
Summary:
The authors from Chiba University, Graduate School of Science and Graduate School of Engineering developed Cu-bpy hybrid polymer-encapsulated magnetic nanobead catalysts (HP-MB) with core-shell structure and magnetic separability, achieving high yields (up to 92%) in aerobic oxidation of silyl enolates to α-hydroxy carbonyl compounds with excellent catalyst recyclability through magnetic separation.
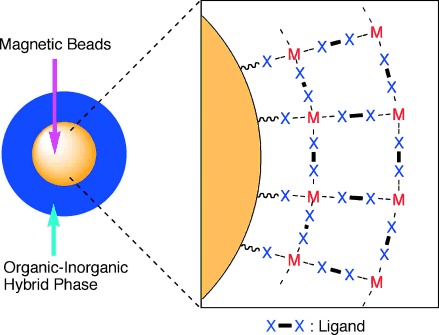
Background:
1. To address the challenge of developing efficient, recyclable heterogeneous catalysts for organic synthesis, previous researchers have explored various supported catalyst systems including superparamagnetic nanoparticle-supported catalysts. However, these existing methods typically require complicated multi-step procedures for incorporating catalytic species onto magnetic supports, limiting their practical applicability and scalability.
2. The authors in this study proposed an innovative in situ self-assembly strategy for constructing organic-inorganic hybrid polymers directly on amino-functionalized magnetic bead surfaces. This simple one-pot method eliminates complex surface modification steps and obtained magnetically separable catalysts with superior catalytic activity compared to free homogeneous counterparts.
Research Content:
1. Synthesis:
The authors synthesized the Cu-bpy HP-MB material using a simple self-assembly method: amino-functionalized magnetic beads (~200 nm diameter, ~130 mmol g⁻¹ NH₂ content, ~40 emu g⁻¹ magnetic susceptibility, purchased from Ademtech SA) were mixed with Cu(BF₄)₂ (10 equivalents to amine content) and 4,4'-bipyridine/bpy (20 equivalents to amine content) in ethanol, followed by gentle agitation at ambient temperature for 8 hours. The excess reagents were removed by washing after magnetic separation. The preparation is extremely simple compared to conventional magnetic-supported catalysts.
2. Characterizations:
1) Surface area analysis: The parent magnetic beads have a specific surface area of 15 m² g⁻¹;
2) SEM tests show the particle size of the material remains approximately 200 nm, with characteristic small projections observed sticking out from the spherical surface after hybrid polymer coating, indicating anisotropic growth of the crystalline Cu-bpy hybrid polymer on the bead surface;
3) ICP analysis revealed that 2 mol% NH₂ functionality on parent beads resulted in 6 mol% Cu atoms incorporated, confirming the formation of a thin organic-inorganic hybrid phase on the surface.
3. Application:
The material was tested in aerobic oxidation of silyl enolates to α-hydroxy carbonyl compounds, and the results are as follows:
- Model substrate (1) converted to α-hydroxy ketone (2) in 92% yield using only 2 mol% catalyst loading at room temperature under 1 atm O₂ in ethanol for 48 hours
- Various cyclic and acyclic ketone substrates were successfully transformed with yields ranging from 75% to >99%
- The catalyst showed excellent recyclability: 1st use (92%), 2nd use (92%), 3rd use (89%), 4th use (85%), 5th use (84%)
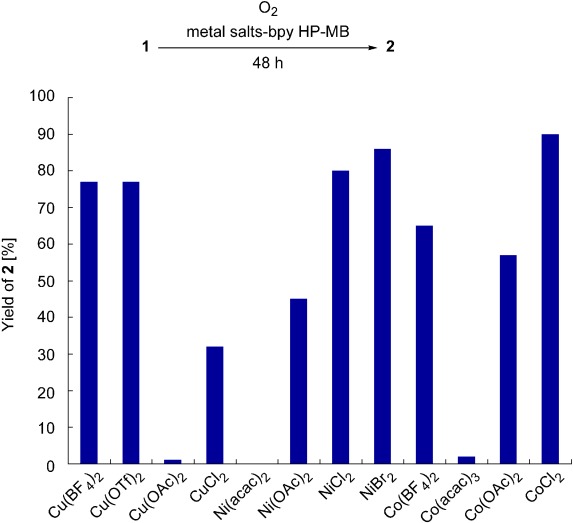
- High-throughput screening (HTS) of different metal-bpy HP-MB catalysts revealed CoCl₂-derived catalyst achieved 90% yield, while NiCl₂ and NiBr₂ showed ~80% and ~85% yields respectively
4. Mechanism:
The analysis of experimental results indicates that:
- The surface-bound hybrid polymer is crucial for catalytic activity: Cu-bpy HP-MB showed higher activity (92% yield) than free homogeneous {[Cu(bpy)(BF₄)₂(H₂O)₂](bpy)}ₙ (53% yield under identical conditions), suggesting the catalytic oxidation occurs on the surface of the hybrid polymer
- The amino-functionalized magnetic bead support provides optimal interface: when amino-methylated polystyrene was used instead, catalytic activity dropped dramatically to only 20% yield, attributed to incorporation of the hybrid polymer into the polymer matrix rather than surface growth
- The magnetic separation mechanism enables rapid catalyst recovery without centrifugation or filtration, with quantitative recovery achieved under external magnetic field
- The molecular oxygen (O₂) serves as the terminal oxidant, making the process environmentally benign and sustainable
Outlook:
This research establishes a facile, generalizable strategy for preparing organic-inorganic hybrid polymer-functionalized magnetic nanobead catalysts through direct self-assembly on amino-functionalized supports. The method eliminates complicated synthetic procedures while delivering catalysts with enhanced activity compared to homogeneous analogues, combined with the practical advantages of magnetic separability and excellent recyclability. The demonstrated high-throughput screening capability opens avenues for rapid discovery of efficient catalytic systems. This platform technology should be extendable to various other metal-ligand combinations for diverse catalytic applications in sustainable chemistry.
Organic–Inorganic Hybrid Polymer-Encapsulated Magnetic Nanobead Catalysts
Authors: Takayoshi Arai, Toru Sato, Hirofumi Kanoh, Katsumi Kaneko, Koichi Oguma, Akira Yanagisawa
DOI: 10.1002/chem.200701371
Link: https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.200701371
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

