Home >
News > Unlocking Inter- to Non-Penetrating Frameworks Using Steric Influences on Spacers for CO₂ Adsorption
Unlocking Inter- to Non-Penetrating Frameworks Using Steric Influences on Spacers for CO₂ Adsorption
Summary:
The authors from the Institute of Materials Research and Engineering (IMRE, A*STAR), National University of Singapore, Soochow University, Fudan University, and University of Malaya developed a series of non-interpenetrating porous coordination frameworks (1-X) with tunable pore sizes and polarizabilities, achieving high CO₂ adsorption capacities (up to 60.2 cm³/g at 273K) and excellent CO₂/N₂ selectivity (up to 111:1) for gas separation applications.
Background:
1. To address the challenge of framework interpenetration in coordination polymers—which significantly reduces pore sizes and limits available framework space—previous researchers have attempted to inhibit catenation using sterically hindered organic linkers with some success. However, systematic studies on the steric influence of halogen functionalities on framework architectures and gas adsorption properties remain limited, and predictive assembly of non-interpenetrating frameworks is still rare due to the complexity and dynamics of assembly processes.
2. The authors in this study proposed an innovative "proximity effect" strategy using bulky substituents (Cl, Br, I, NO₂) on 1,4-benzenedicarboxylate spacers to influence coordination modes, successfully unlocking interpenetrating frameworks to give isostructural non-penetrating structures with enhanced porosity and CO₂ adsorption performance.
Research Content:
1. Synthesis:
The authors synthesized four isostructural complexes {Cu₂(2-X-BDC)₂(Bpe)₂·3DMSO}n (1-X, where X = Cl, Br, I, NO₂) using a solvothermal method. The synthesis involved reacting Cu(NO₃)₂·6H₂O, 2-X-H₂BDC ligands (2-Cl-H₂BDC, 2-Br-H₂BDC, 2-I-H₂BDC, 2-NO₂-H₂BDC), and Bpe (1,2-bis(4-pyridyl)ethylene) in a mixed DMF/DMSO solvent (1:1 v/v) at 120°C for 24 hours. Guest-free frameworks (1-X') were obtained by soaking in acetone for 3 weeks followed by heating at 140°C under vacuum for 48 hours.
2. Characterizations:
1) Gas Sorption Analysis (BET and pore size distribution): N₂ sorption at 77K showed type II isotherms indicating permanent microporosity. CO₂ sorption at 273K and 298K (up to 1 atm) showed type I isotherms. Pore size distributions calculated using DFT revealed pore sizes decreasing with increasing substituent bulkiness: 1-Cl' (8.5 Å) > 1-Br' (8.0 Å) > 1-I' (7.9 Å) > 1-NO₂' (7.7 Å) (Figure S20).
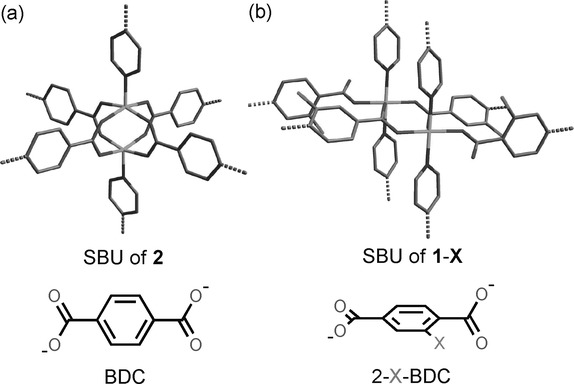
2) PXRD and Single-Crystal XRD: Confirmed isostructural nature of all 1-X complexes in monoclinic C2/c space group. The structures revealed 1D channels with pore dimensions of 9×11 Å, significantly larger than the interpenetrated framework 2 (<5×5 Å).
3) TGA Analysis: Showed loss of all DMSO molecules at ~220°C, confirming thermal stability of the frameworks (Figures S9–S10).
4) IR Spectroscopy: Confirmed successful incorporation of different substituents (Figure S7).
3. Application:
The material was tested for CO₂ capture and CO₂/N₂ separation. At 273K and 1 atm, CO₂ adsorption capacities were: 1-Cl' (60.2 cm³/g), 1-I' (18.5 cm³/g), 1-Br' (16.3 cm³/g), 1-NO₂' (14.5 cm³/g), compared to only 10.9 cm³/g for the interpenetrated framework 2'. The CO₂/N₂ selectivity for 1-Cl' reached 111:1 (v:v) at 273K and 0.1 atm, and 68:1 at 298K and 0.1 atm, surpassing many reported MOF materials including MOF-74-Mg (12:1).
4. Mechanism:
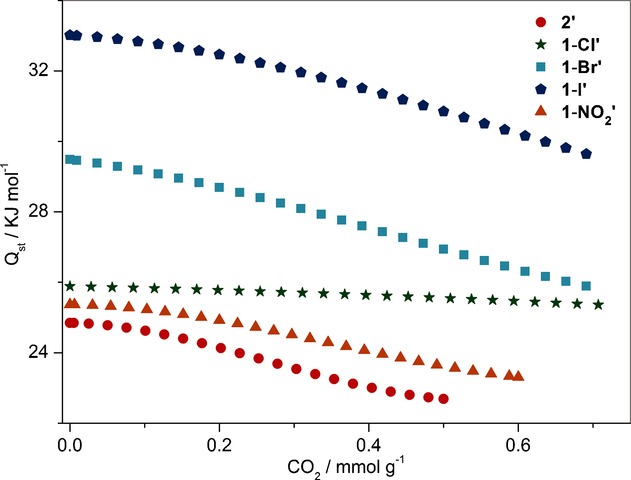
The analysis of experimental results revealed that introduction of ortho-substituents on the BDC linker causes rotation of the adjacent carboxyl plane to be perpendicular to the benzene ring, altering the secondary building unit (SBU) from a paddle-wheel (in framework 2) to a dinuclear unit with mixed coordination modes (μ₂-η¹-η¹ and μ₁-η¹). This structural change unlocks the interpenetration, creating larger 1D channels. The diverging CO₂ adsorption performances among 1-X' series are attributed to two factors: (1) pore size effects—1-Cl' has the largest pore size enabling highest CO₂ uptake; and (2) polarizability effects—heavier halogens (Br, I) increase framework-CO₂ interactions (higher Qst values: 29.5 kJ/mol for 1-Br', 33.0 kJ/mol for 1-I'), but their smaller pore sizes limit overall capacity. The optimal balance is achieved with 1-Cl', combining large pore volume with moderate host-guest interactions (Qst = 25.9 kJ/mol). The high CO₂/N₂ selectivity arises from the larger quadrupole moment and higher polarizability of CO₂ compared to N₂, combined with the thermodynamic equilibrium effect.
Outlook:
This research demonstrates a simple and practical molecular engineering strategy at spacer units to control framework interpenetration and tune gas adsorption properties. By systematically varying halogen substituents, the authors achieved non-interpenetrating frameworks with superior CO₂ capture performance and high selectivity over N₂. This approach provides valuable insights for designing next-generation porous materials for carbon capture and gas separation applications, with potential for extension to other substituent types and framework systems.
Unlocking Inter- to Non-Penetrating Frameworks Using Steric Influences on Spacers for CO₂ Adsorption
Authors: Sheng-Li Huang, Wen-Hua Zhang, Yun Ling, Seik Weng Ng, He-Kuan Luo, T.S. Andy Hor
DOI: 10.1002/asia.201500231
Link: https://onlinelibrary.wiley.com/doi/10.1002/asia.201500231
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

