Home >
News > Molecular Sieving of Ethane from Ethylene through the Molecular Cross-Section Size Differentiation in Gallate-based Metal–Organic Frameworks
Molecular Sieving of Ethane from Ethylene through the Molecular Cross-Section Size Differentiation in Gallate-based Metal–Organic Frameworks
Summary:
The authors from Zhejiang University, University of Texas at San Antonio, and NIST Center for Neutron Research developed a family of gallate-based metal-organic frameworks (M-gallate, M = Ni, Mg, Co) with precisely tuned aperture sizes (3.47–3.69 Å) featuring 3D interconnected zigzag channels, achieving unprecedented molecular sieving of ethylene from ethane with IAST selectivity up to 52 and high ethylene uptake of 3.37 mmol/g at 298 K and 1 bar, outperforming state-of-the-art MOF materials.
Background:
1. To address the challenge of energy-intensive cryogenic distillation for ethylene/ethane separation (accounting for >0.3% of global energy consumption), previous researchers developed π-complexation adsorbents using Ag+/Cu+ ions and MOFs with open metal sites (OMSs) such as MMOF-74, achieving success in selective ethylene capture; however, these systems suffer from strong binding making ethylene recovery difficult, potential explosive hazards, and unavoidable co-adsorption of ethane due to polarization effects.
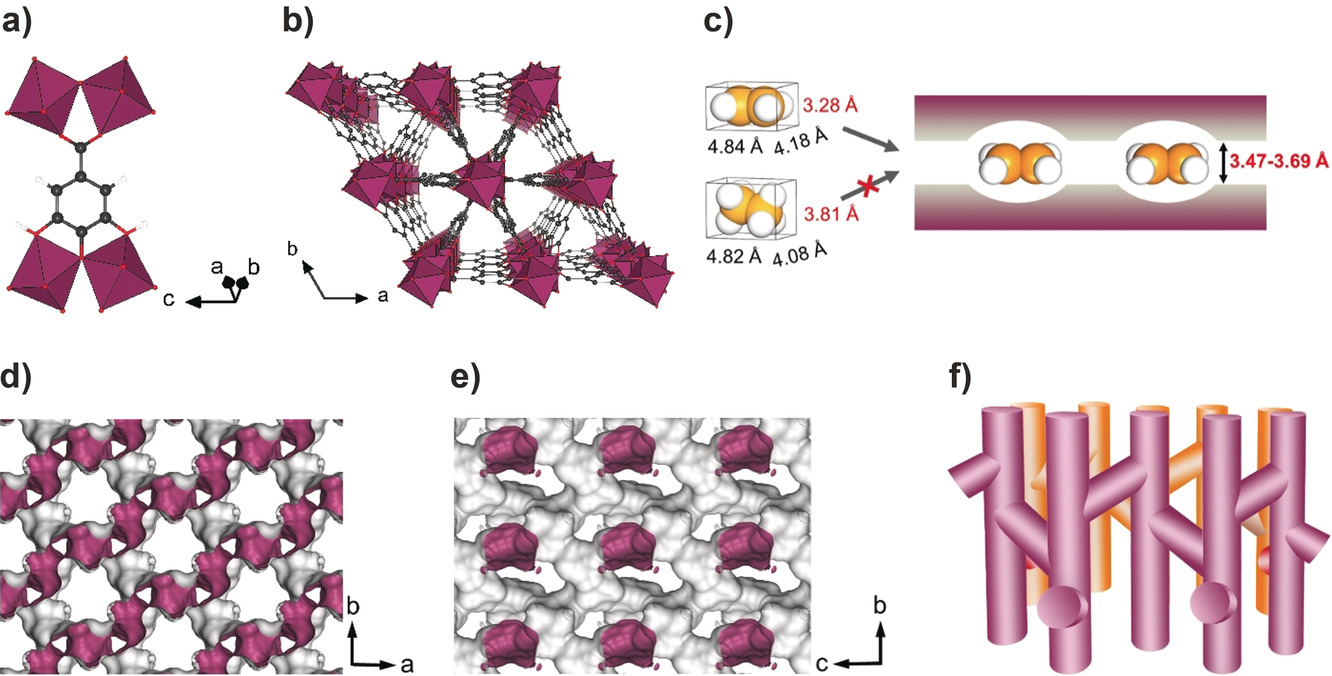
2. The authors in this study proposed an innovative method utilizing molecular cross-section size differentiation rather than kinetic diameter-based sieving, designing gallate-based MOFs with aperture sizes between the minimum cross-section of ethylene (3.28×4.18 Ų) and ethane (3.81×4.08 Ų), and obtained benchmark separation performance with high selectivity, uptake capacity, and excellent stability.
Research Content:
1. Synthesis:
The authors synthesized M-gallate (M = Ni, Mg, Co) using a solvothermal method by reacting metal chlorides (NiCl₂·6H₂O, MgCl₂, CoCl₂·6H₂O) with gallic acid monohydrate in KOH aqueous solution at 120°C for 24 hours, followed by activation at 120°C under ultrahigh vacuum. Scale-up synthesis was achieved by refluxing at 80°C under ambient pressure.
2. Characterizations:
1) Results of BET surface areas of 424, 559, and 475 m²/g for Ni-, Mg-, and Co-gallate respectively, determined from CO₂ adsorption isotherms at 195 K; pore size distribution confirmed narrow window apertures of 3.47×4.85 Ų (Ni), 3.56×4.84 Ų (Mg), and 3.69×4.95 Ų (Co);
2) Single-crystal X-ray diffraction revealed P3₂21 space group structures with infinite chains of corner-sharing distorted MO₆ octahedra connected through gallate ligands, forming quasi-discrete fusiform branched channels;
3) Neutron powder diffraction experiments at 200 K using C₂D₄-loaded Mg-gallate identified three distinct binding sites with cooperative C···H–O supramolecular interactions (2.28–2.68 Å) and C–D···O hydrogen bonding.
3. Application:
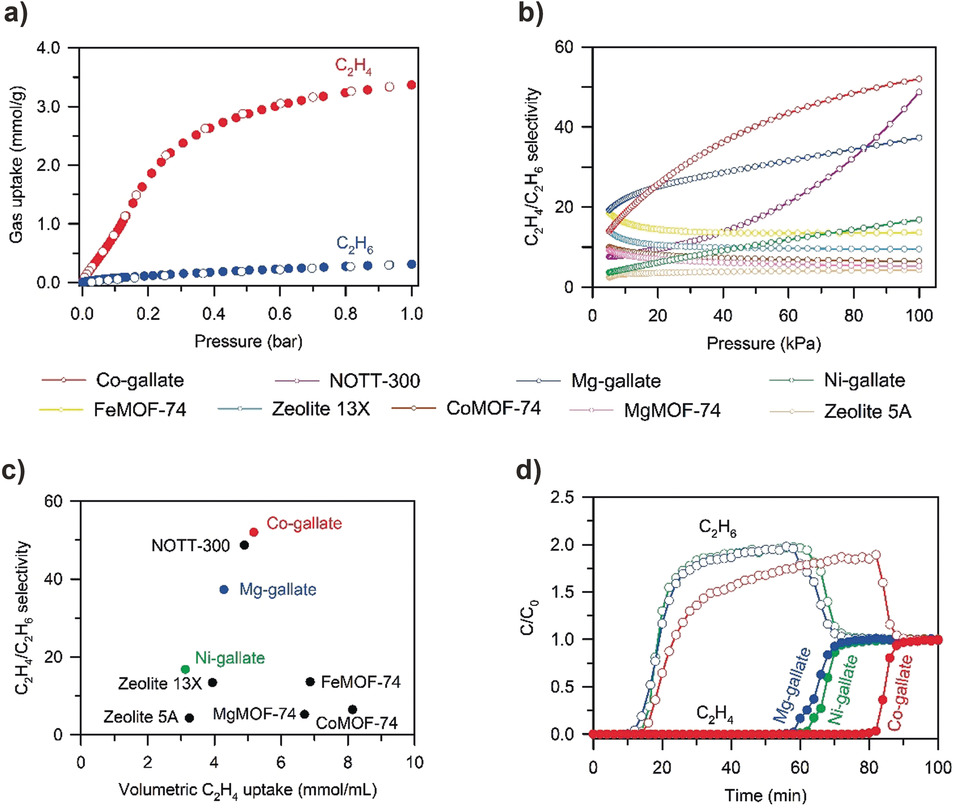
The material was tested for ethylene/ethane separation applications. Co-gallate achieved an unprecedented IAST selectivity of 52 for C₂H₄/C₂H₆ at 298 K and 1 bar with ethylene uptake of 3.37 mmol/g (5.18 mmol/mL volumetric). Breakthrough experiments with equimolar mixtures demonstrated complete separation with ethane breaking through immediately while ethylene was retained for >55 minutes. Mg-gallate showed excellent recyclability over 10 cycles, water stability for 3 weeks at 75% humidity, and maintained performance after scale-up synthesis and extrusion with hydroxypropyl cellulose binder.
4. Mechanism:
the analysis of the experiment result showed that the molecular sieving mechanism operates through cross-section size differentiation rather than kinetic diameter exclusion. The aperture sizes (3.47–3.69 Å) are smaller than ethane's minimum cross-section (3.81 Å) but larger than ethylene's (3.28 Å), enabling selective ethylene passage. Neutron diffraction revealed ethylene molecules adsorb at channel intersections through multiple cooperative supramolecular interactions, with isosteric heats of adsorption of 32–44 kJ/mol indicating moderate binding strength favorable for regeneration.
Outlook:
This research establishes M-gallate as a new benchmark material for ethylene/ethane separation, demonstrating the first successful application of molecular cross-section size differentiation in MOFs. The combination of high selectivity, capacity, water stability, and scalable synthesis from inexpensive biomass-derived gallic acid positions Mg-gallate as a promising candidate for industrial pressure-swing adsorption processes, offering significant energy savings compared to cryogenic distillation.
Molecular Sieving of Ethane from Ethylene through the Molecular Cross-Section Size Differentiation in Gallate-based Metal–Organic Frameworks
Authors: Zongbi Bao, Jiawei Wang, Zhiguo Zhang, Huabin Xing, Qiwei Yang, Yiwen Yang, Hui Wu, Rajamani Krishna, Wei Zhou, Banglin Chen, Qilong Ren
DOI: 10.1002/anie.201808716
Link: https://doi.org/10.1002/anie.201808716
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

