Home >
News > Postsynthetic Covalent Modification of a Neutral Metal-Organic Framework
Postsynthetic Covalent Modification of a Neutral Metal-Organic Framework
Summary:
The authors from Department of Chemistry and Biochemistry, University of California, San Diego developed IRMOF-3-AM1 (modified IRMOF-3) with retained cubic topology and thermal stability, achieving successful postsynthetic covalent modification of neutral metal-organic frameworks (MOFs) in the field of functional MOF preparation.
Background:
1. To address the limitation that functional groups in MOFs must be compatible with solvothermal synthesis conditions (e.g., thermal stability, non-coordination), previous researchers synthesized MOFs via solvothermal complexation, yet only one case of direct covalent modification on crystalline MOF lattices was reported.
2. The authors in this study proposed an innovative postsynthetic covalent modification method (analogous to protein posttranslational modification) and obtained IRMOF-3-AM1 with >80% acetylation conversion while maintaining MOF structural integrity.
Research Content:
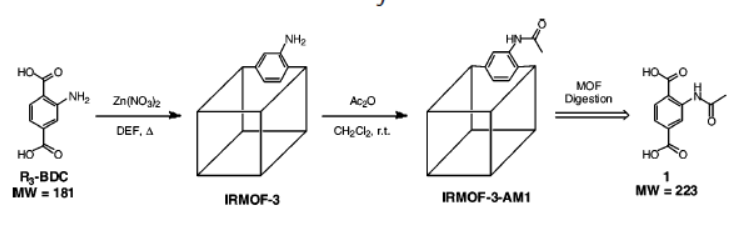
1. Synthesis:
The authors first synthesized IRMOF-3 via solvothermal method using Zn(NO₃)₂·4H₂O and 2-amino-1,4-benzene dicarboxylic acid (R₃-BDC); then modified IRMOF-3 by suspending 27 mg dried IRMOF-3 in 1.00 mL CH₂Cl₂, adding 20 μL acetic anhydride, reacting at room temperature for hours to days, followed by washing, soaking, and vacuum drying at 70 °C for ~10 h to obtain IRMOF-3-AM1.
2. Characterizations:
1) BET and pore size distribution: Not tested in the literature.
2) SEM/TEM tests: Not conducted in the literature.
3) Other tests:
- ESI-MS (negative mode): Detected base peak at m/z 222 ([1-H]⁻, modified ligand).
- ¹H NMR: New resonances at 2.10, 7.60, 8.05, 9.07 ppm; >80% acetylation conversion after 5 days.
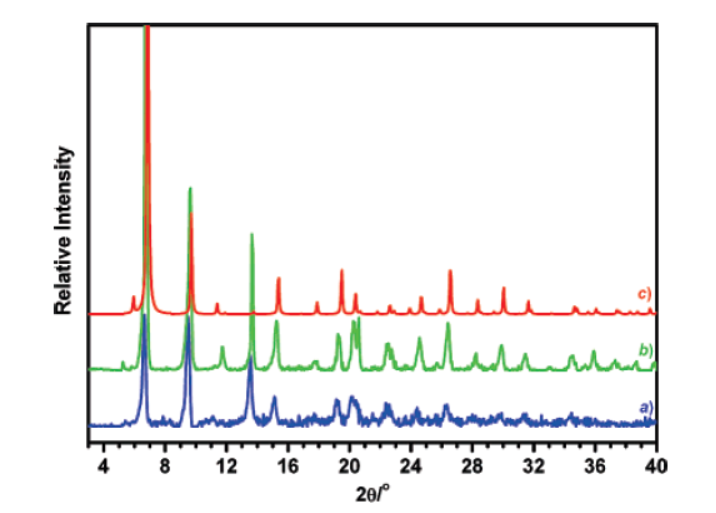
- PXRD: IRMOF-3-AM1 showed consistent cubic lattice with as-synthesized and simulated IRMOF-3.
- TGA (20 °C/min under N₂): IRMOF-3-AM1 had thermal stability comparable to IRMOF-3.
- Single-crystal XRD: Space group Fm-3m, a=b=c=25.8074(18) Å, V=17188(2) ų, Z=8.
3. Application:
Not tested in the literature.
4. Mechanism:
Analysis of experiment results (NMR monitoring, control experiments) showed the reaction was heterogeneous—IRMOF-3 lattice remained intact, no free R₃-BDC/modified ligand in solution; the amino group on R₃-BDC in IRMOF-3 reacted with acetic anhydride via acetylation to form amide groups, confirming postsynthetic modification feasibility.
Outlook:
This research successfully realized postsynthetic covalent modification of neutral MOFs, breaking traditional solvothermal synthesis limitations, providing a new strategy for preparing functional MOFs, and laying a foundation for modifying MOF pores and stabilizing MOF nanoparticles.
Postsynthetic Covalent Modification of a Neutral Metal-Organic Framework
Authors: Zhenqiang Wang, Seth M. Cohen*
DOI: 10.1021/ja074366o
Link: https://pubs.acs.org/doi/10.1021/ja074366o
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

