Home >
News > Metal-Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature
Metal-Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature
Summary:
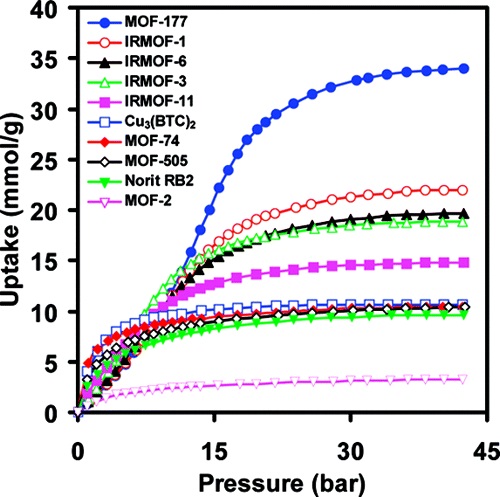
The authors from Materials Design and Discovery Group, Department of Chemistry, University of Michigan developed 9 metal-organic frameworks (MOFs) including MOF-177, IRMOF-1/3/6/11 with high porosity and tunable structures, achieving exceptional CO₂ storage capacity at room temperature (MOF-177: 33.5 mmol/g at 35 bar).
Background:
1. To address high cost/inefficiency of traditional CO₂ removal (chilling/pressurizing, aqueous amine adsorption) and low capacity of benchmark materials (zeolite 13X: 7.4 mmol/g; MAXSORB carbon: 25 mmol/g), previous researchers used oxide chemisorption or porous material physisorption but lacked reversibility and molecular-level tuning.
2. The authors proposed using MOFs with ordered structures, high thermal stability, and adjustable functionality, systematically testing 9 MOFs to optimize CO₂ storage, achieving higher capacity and tunable adsorption performance.
Research Content:
1.Synthesis:
MOFs were synthesized via solvothermal reaction (e.g., MOF-177: Zn(NO₃)₂·6H₂O + H₃BTB in DEF at 100℃ for 20 h), followed by solvent exchange (CHCl₃/DMF) and vacuum activation (60-270℃, 6-24 h) to remove guests.
2.Characterizations:
1) BET: MOF-177 (4508 m²/g, 1.75 cm³/g), IRMOF-1 (2833 m²/g, 1.13 cm³/g), pore sizes 5-17 Å.
2) SEM/TEM tests: Not mentioned in the documents.
3) PXRD confirmed crystalline structure; TGA showed stability (most stable up to 300℃); buoyancy-corrected gravimetry measured CO₂ uptake.
3.Application:
In room-temperature CO₂ storage, MOF-177’s volumetric capacity was 9x that of empty containers, 2x that of zeolite 13X/MAXSORB. IRMOFs showed sigmoidal isotherms, with step pressures tailored by pore size/functional groups.
4.Mechanism:
CO₂ uptake correlates with MOF surface area/pore volume; amino groups in IRMOF-3 enhance CO₂ interaction (H-bonding/lone-pair interaction); larger pores (MOF-177: 11×17 Å) enable higher capacity; step pressures relate to pore size for efficient adsorption at target pressures.
Outlook:
This research identifies MOFs as superior CO₂ storage materials, clarifies structure-adsorption relationships, and enables tailored MOF design for flue gas CO₂ removal, advancing low-cost, high-efficiency carbon capture technology.
Metal-Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature
Authors: Andrew R. Millward, Omar M. Yaghi
DOI: 10.1021/ja0570032
Link: https://pubs.acs.org/doi/10.1021/ja0570032
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

