Home >
News > Rapid Cycling, High-Yield MOF Water Harvester
Rapid Cycling, High-Yield MOF Water Harvester
Summary:
The authors from the University of California-Berkeley, Lawrence Berkeley National Laboratory, Kavli Energy NanoSciences Institute, Berkeley Global Science Institute, University of South Alabama, and UC Berkeley-KACST Joint Center of Excellence for Nanomaterials for Clean Energy Applications developedMOF-303 (a microporous aluminum-based metal-organic framework, Al(OH)(PZDC), PZDC = 1H-pyrazole-3,5-dicarboxylate) with fast adsorption-desorption kinetics, low isosteric heat of adsorption (~52 kJ·mol⁻¹), and high hydrolytic stability. It achieved exceptional results inatmospheric water harvesting, with productivities of 1.3 L·kgᵢₒբ⁻¹·day⁻¹ in indoor arid environments (32% RH, 27 °C) and 0.7 L·kgᵢₒբ⁻¹·day⁻¹ in the Mojave Desert (10% RH, 27 °C).
Background:
1. To address global water scarcity (projected 50% population water stress by 2050) and unfeasible direct air water extraction in arid regions, previous researchers developed sorbent-assisted water harvesting technologies using zeolites and MOFs. These technologies only performed 1 water harvesting cycle (WHC) per day, resulting in low productivity that failed to meet demand.
2. The authors proposed focusing on sorbents with rapid water sorption dynamics (instead of only high water capacity) and used MOF-303 to design a new water harvester. This enabled multiple WHCs per day, improving desert-operated device productivity by 1 order of magnitude.
Research Content:
1. Synthesis
-MOF-303 Synthesis: 3,5-pyrazoledicarboxylic acid monohydrate (7.50 g, 43.1 mmol) was dissolved in deionized H₂O (725 mL) and LiOH solution (2.57 M, 25 mL), heated at 120 °C for 30 min. AlCl₃·6H₂O (10.4 g, 43.1 mmol) was added, sonicated to dissolve precipitates, and heated at 100 °C for 15 h. The product was filtered, washed with water, then MeOH (Soxhlet extraction, 24 h), air-dried (3 days), and activated under dynamic vacuum (~10⁻³ mbar) at 150 °C for 6 h (yield: 3.6 g).
-Al-Fumarate Synthesis: Fumaric acid (66.7 mg, 0.575 mmol) and AlCl₃·6H₂O (139 mg, 0.575 mmol) were dissolved in KOH solution (0.086 M, 10 mL), incubated at 100 °C for 12 h. The product was washed with H₂O (5 times/day, 2 days) and MeOH (5 times/day, 2 days).
-Commercial Sorbents: SAPO-34 (ACS Material, LLC) and zeolite 13X (Alfa Aeser) were purchased, activated under dynamic vacuum (~10⁻³ mbar) at 180 °C (12 h) and 225 °C (12 h), respectively.
2. Characterizations
1.BET and Pore Size Distribution (argon sorption, 87 K):
- MOF-303: BET surface area 1119 m²·g⁻¹, pore volume 0.580 cm³·g⁻¹;
- Al-fumarate: BET surface area 1080 m²·g⁻¹, pore volume 0.574 cm³·g⁻¹;
- SAPO-34: BET surface area 931 m²·g⁻¹, pore volume 0.398 cm³·g⁻¹;
- Zeolite 13X: BET surface area 1077 m²·g⁻¹, pore volume 0.393 cm³·g⁻¹.
2.SEM Tests (FEI Quanta 3D FEG, 10 kV): MOF-303, Al-fumarate, SAPO-34, and zeolite 13X all had crystallite sizes of 2–5 μm (after synthesis, washing, activation).
3.Other Tests:
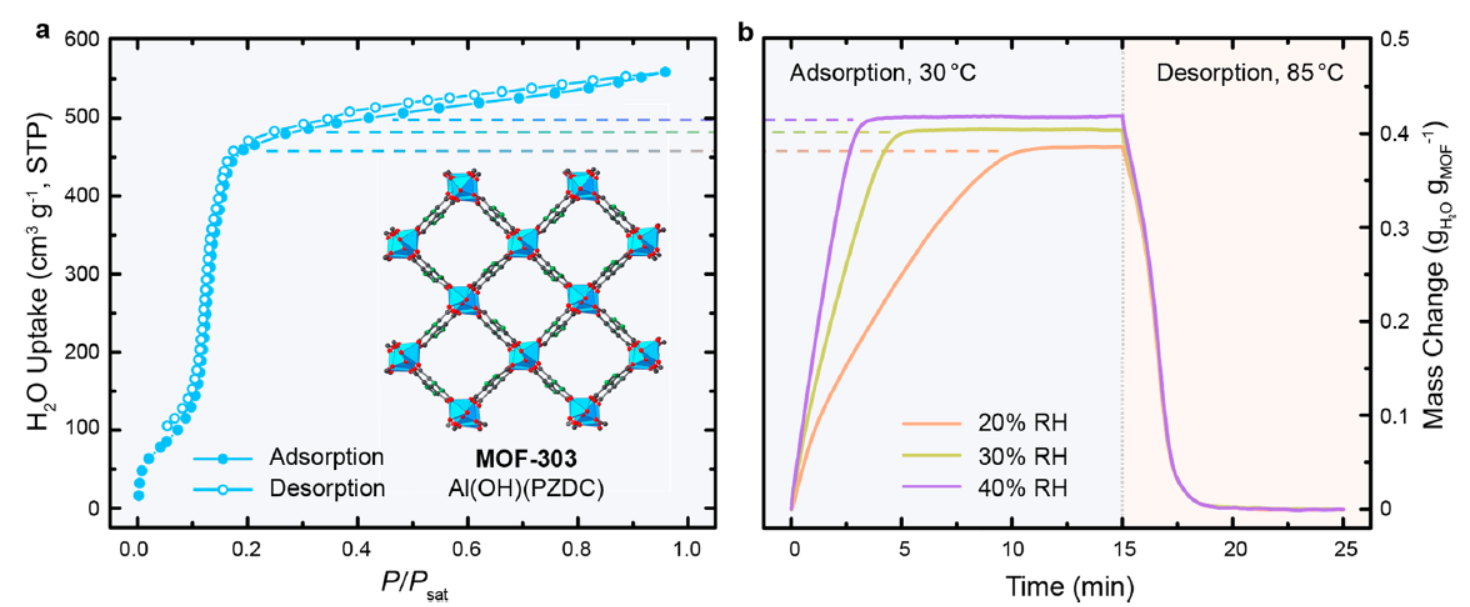
-Water Sorption Isotherms (BEL Japan BELSORP-aqua3): MOF-303 had 39 wt% water uptake at 20% RH (30 °C) with minimal hysteresis;
-Isosteric Heats of Adsorption (Clausius-Clapeyron relation): MOF-303 ~52 kJ·mol⁻¹, Al-fumarate and SAPO-34 50–54 kJ·mol⁻¹ (zeolite 13X unmeasurable due to isotherm overlap);
-PXRD (Bruker D8 Advance, Cu Kα1): Confirmed crystalline structures of all materials;
-Density (helium pycnometer): MOF-303 skeletal density 1.77 g·cm⁻³, powder particle density 0.873 g·cm⁻³; Al-fumarate skeletal density 1.66 g·cm⁻³, powder particle density 0.850 g·cm⁻³.
3. Application
-Indoor Arid Test: Harvester with 433 g MOF-303 operated 24 h (9 WHCs) at 27 ± 1 °C, 32% RH, producing 1.3 L·kgᵢₒբ⁻¹·day⁻¹; with Al-fumarate (same mass) at 23 ± 1 °C, 32% RH, produced 0.55 L·kgᵢₒբ⁻¹·day⁻¹.
-Mojave Desert Test: Solar-powered (265 W PV module + 4×12 V batteries) harvester operated 3 days, producing 0.7 L·kgᵢₒբ⁻¹·day⁻¹ (functioned at -4 °C dew point, outside direct condensation range).
-Dynamic Sorption: MOF-303 saturated in 3–10 min (30 °C, 20–40% RH) and desorbed completely in ~1 h (85 °C); zeolite 13X required >10 h to desorb at 120 °C.
4. Mechanism
-Fast Sorption: MOF-303’s hydrophilic PZDC linker and rod-like Al-based secondary building units (SBUs) accelerate water molecule diffusion/adsorption; moderate Qₛₜ enables mild heating (85–100 °C) for desorption.
-Device Efficiency: Cross-flow exchanger with laminar air flow and packing porosity ~0.7 (matching TGA tests) ensures efficient mass/heat transfer; condenser (Rigid HVAC DV1910E-1C 12V Pro) has ~85% condensation efficiency.
-Stability: MOF-303 shows no capacity loss after 150 cycles, due to hydrolytically stable Al-SBU structure.
Outlook:
This research confirms that rapid sorption dynamics are critical for high-productivity water harvesting. MOF-303 and the harvester design validate sorbent-assisted atmospheric water harvesting as a viable solution for arid-region water scarcity, supporting scalable off-grid water production.
Rapid Cycling and Exceptional Yield in a Metal-Organic Framework Water Harvester
Authors : Nikita Hanikel, Mathieu S. Prévot, Farhad Fathieh, Eugene A. Kapustin, Hao Lyu, Haoze Wang, Nicolas J. Diercks, T. Grant Glover, Omar M. Yaghi
DOI : 10.1021/acscentsci.9b00745
Link: https://pubs.acs.org/doi/10.1021/acscentsci.9b00745-Supporting Information
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

