Home >
News > Cost-Effective Scalable Production of Ultra-Stable Alkyl MOF Featuring Single-Molecule C₃H₆ Trap for Record Capture of Trace C₃H₆ from C₂H₄
Cost-Effective Scalable Production of Ultra-Stable Alkyl MOF Featuring Single-Molecule C₃H₆ Trap for Record Capture of Trace C₃H₆ from C₂H₄
Summary:
The authors from Chuzhou University, Hebei Normal University of Science and Technology, PetroChina Petrochemical Research Institute, and Beijing University of Chemical Technology developed an ultra-stable alkyl MOF (Al-CDC) with a single-molecule C₃H₆ trap structure, achieving record-breaking results in trace C₃H₆ capture from C₂H₄ in the petrochemical separation field.
Background:
1. To address the difficulty of deep removal of trace C₃H₆ from C₂H₄ (due to their similar physicochemical properties) and the high energy consumption of industrial extraction distillation, previous researchers developed MOF adsorbents (e.g., Al-BDC, ZJU-74-Pd), which showed some separation effects but had problems like low trace C₃H₆ adsorption capacity, high synthesis cost, and poor scalability.
2. The authors proposed an innovative strategy to construct a single-molecule C₃H₆ trap in Al-CDC, realizing low-cost scalable synthesis and excellent trace C₃H₆ capture performance.
Research Content:
1. Synthesis:
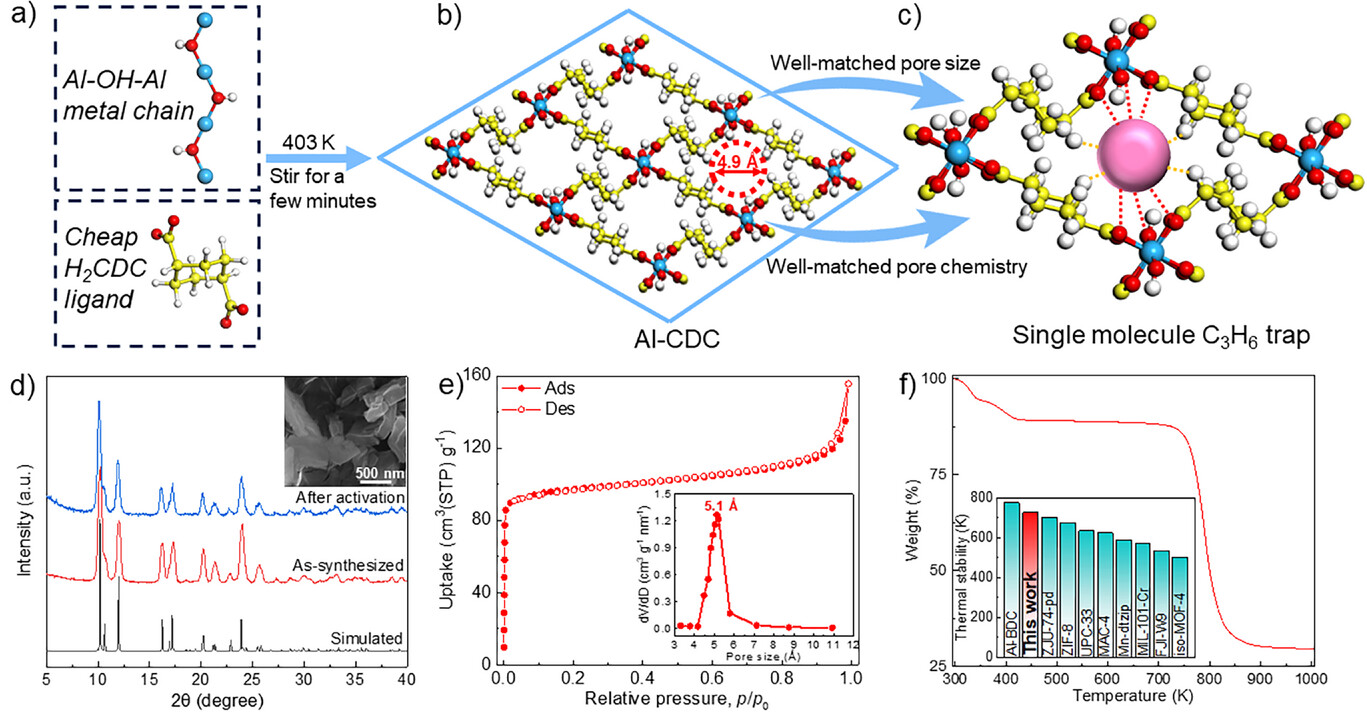
The authors synthesized Al-CDC via a reflux method: AlCl₆·6H₂O and trans-1,4-cyclohexanedicarboxylic acid (H₂CDC) were mixed in DMF/H₂O, stirred at 403 K for several minutes, then washed and dried; large-scale synthesis (0.3 mol raw materials) yielded 63.4 g product with 87.6% yield.
2. Characterizations:
1) BET: Specific surface area 392.4 m²/g, pore volume 0.15 cm³/g; pore size distribution: 5.1 Å (ultramicropore, NLDFT model).
2) SEM tests show the material has uniform hexagonal rod-like morphology; no specific particle size value was provided.
3) TGA: Thermal decomposition temperature 725 K; PXRD: Sharp peaks, consistent with simulated triclinic P1 space group structure; FTIR: Confirmed Al³⁺-H₂CDC complexation, with C₃H₆ adsorption-induced hydrogen bond peaks (3717-3590 cm⁻¹).
3. Application:
- Trace C₃H₆ capture from C₂H₄: At 298 K, 0.01 bar, C₃H₆ adsorption capacity 40.8 cm³(STP)g⁻¹ (record); Henry coefficient 12685.5 cm³(STP)g⁻¹bar⁻¹; C₃H₆/C₂H₄ selectivity 16.3.
- Dynamic breakthrough test: For C₃H₆/C₂H₄ (1/99) mixture, produced ≥99.999% C₂H₄ with productivity 410.5 cm³(STP)g⁻¹; stable after 10 cycles.
4. Mechanism:
- Experimental analysis: In-situ PXRD and FTIR confirmed C₃H₆ was trapped at the pore center of Al-CDC.
- Theoretical calculation: DFT showed C₃H₆ formed strong hydrogen bonds (2.423-2.600 Å) with dual O of μ₂-OH groups and multiple van der Waals forces (2.572-2.904 Å) with aliphatic H, resulting in high binding energy (52.5 kJ mol⁻¹); C₂H₄ only had weak van der Waals forces (binding energy 29.7 kJ mol⁻¹), ensuring selective capture.
Outlook:
This research successfully developed Al-CDC with a single-molecule C₃H₆ trap, solving the trade-off between MOF separation performance, stability, and scalability. It provides a new design paradigm for high-efficiency adsorbents and promotes the application of adsorption technology in low-energy petrochemical separation.
Cost-Effective Scalable Production of Ultra-Stable Alkyl MOF Featuring Single-Molecule C₃H₆ Trap for Record Capture of Trace C₃H₆ from C₂H₄
Authors: Miao Chang, Zitong Wang, Ruihan Wang, Minghui Liu, Yujie Wang, Dahuan Liu
DOI: 10.1002/anie.202515496
Link: https://onlinelibrary.wiley.com/doi/full/10.1002/anie.202515496
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

