Home >
News > Green separation of rare earth elements by valence-selective crystallization of MOFs
Green separation of rare earth elements by valence-selective crystallization of MOFs
Summary:
The authors from Key Laboratory for Ultrafine Materials of Ministry of Education, School of Materials Science and Engineering, East China University of Science and Technology (Shanghai, China) developed a valence-selective crystallization (VSC) strategy and multiple Ce-based metal–organic frameworks (Ce-MOFs, including Ce-MOF-801, Ce-UiO-66, Ce-MOF-808, Ce-UiO-66-SUC) with characteristics of fast synthesis, high crystallinity, and good porosity. They achieved high-efficiency (99.97% purity for Ce⁴⁺), green, and scalable separation results in the application of rare earth (RE) element separation (especially Ce from RE mixtures) and Fe³⁺ separation from mixed metal ions.
Background:
1. To address the problem of difficult and environmentally unfriendly separation of Ce from RE mixtures (due to similar physical and chemical properties of RE elements, and high cost/pollution of traditional solvent extraction), previous researchers developed methods such as solvent extraction, ion chromatography, and solid adsorption. These methods have certain separation effects but lack convenience, short separation time, low cost, high efficiency, and environmental benignity.
2. The authors proposed an innovative VSC strategy based on the selective crystallization of Ce(IV) into pre-designed MOFs. They realized aqueous-phase, mild synthesis of Ce-MOFs and achieved high-purity, fast, and scalable Ce separation, with the strategy also extensible to Fe³⁺ separation.
Research Content:
1. Synthesis
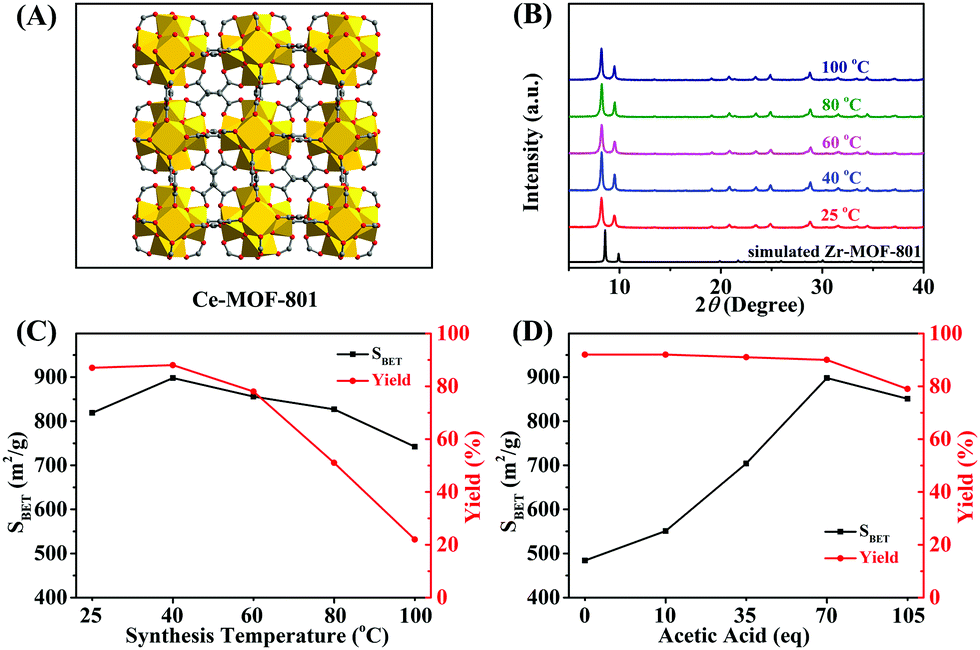
-Ce-MOF-801 synthesis: Using water as solvent, fumaric acid (FUM) as ligand, and Ce(NH₄)₂(NO₃)₆ as Ce source, the authors synthesized Ce-MOF-801 via aqueous-phase reaction. They optimized conditions: reaction temperature 40 °C, reaction time 6 h, and 70 eq acetic acid (HAc) as modulator. For rapid separation, it could also be synthesized at 25 °C in 10 min.
-Other Ce-MOFs synthesis: Using sodium 1,4-benzenedicarboxylate (Na₂BDC), 1,3,5-benzenetricarboxylic acid (BTC), and succinic acid (SUC) as ligands respectively, the authors synthesized Ce-UiO-66, Ce-MOF-808, and Ce-UiO-66-SUC via aqueous-phase reaction at 25 °C in 10 min.
-MIL-100(Fe) synthesis: Using BTC as ligand and Fe(NO₃)₃·9H₂O as Fe source, the authors synthesized MIL-100(Fe) via hydrothermal reaction at 95 °C for 12 h to separate Fe³⁺.
2. Characterizations
1.BET and pore size distribution:
- Ce-MOF-801 (optimized) had a BET surface area of 898 m²/g; at 25 °C for 10 min, it was 763 m²/g. RE/Ce-MOF-801 (with La, Pr, etc.) had BET surface areas around 790–813 m²/g.
- Ce-UiO-66, Ce-MOF-808, and Ce-UiO-66-SUC had pure phase structures (verified by PXRD), indicating good porosity.
- MIL-100(Fe) had a pure phase structure, with high Fe molar fraction (99.956%) in the product.
2.SEM tests: SEM images (Fig. S7) showed that with 105 eq HAc, Ce-MOF-801 particle size increased, leading to decreased external surface area (95 m²/g) compared to 70 eq HAc (131 m²/g).
3.Other tests:
- PXRD patterns confirmed that all synthesized MOFs had pure phases (matching simulated patterns), and trivalent RE ions or other metal ions did not affect MOF phase purity.
- ICP-OES results showed that Ce molar fraction in RE/Ce-MOF-801 was over 99.97%, and La molar fraction was less than 0.03%; Fe molar fraction in MIL-100(Fe) was 99.956%.
3. Application
-Ce separation from RE mixtures: When separating Ce from 7 RE ions (La³⁺, Pr³⁺, Nd³⁺, Sm³⁺, Tb³⁺, Er³⁺, Yb³⁺) at 25 °C for 10 min, Ce purity in RE/Ce-MOF-801 exceeded 99.97%. Enlarging the system 100-fold (obtaining 28 g La/Ce-MOF-801) still maintained La molar fraction < 0.03%.
-Fe³⁺ separation from mixed metal ions: Separating Fe³⁺ from Fe³⁺/Cu²⁺/Ni²⁺/Co²⁺ mixtures, Fe molar fraction in MIL-100(Fe) reached 99.956%, with trace amounts of other ions.
4. Mechanism
- The core mechanism of the VSC strategy relies on the valence difference between Ce(IV) and other RE³⁺. Ce(IV) has a strong crystallization ability with ligands (FUM, Na₂BDC, etc.) to form stable Ce-MOFs, while RE³⁺ cannot form such MOFs and only has weak physical adsorption (resulting in <0.03% molar fraction in Ce-MOFs).
- For Fe³⁺ separation, Fe³⁺ specifically coordinates with BTC to form MIL-100(Fe), while divalent ions (Cu²⁺, Ni²⁺, Co²⁺) cannot, achieving selective separation.
Outlook:
This research innovatively developed the VSC strategy, realizing green, fast, high-efficiency, and scalable Ce separation from RE mixtures. It also expanded the strategy to Fe³⁺ separation, providing a new approach for RE purification and other metal ion separation. The wide ligand selection (FUM, Na₂BDC, BTC, SUC) and aqueous-phase synthesis make it promising for industrial applications, promoting the development of green separation technology in the chemical materials field.
Green separation of rare earth elements by valence-selective crystallization of MOFs
Authors: Pengfei Yang, Qixin Zhuang, Yongsheng Li, Jinlou Gu
DOI: 10.1039/c9cc07849e
Link: https://pubs.rsc.org/en/content/articlelanding/2019/cc/c9cc07849e
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

