Home >
News > Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs
Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs
Summary:
The authors from College of Environmental Science and Engineering, Hunan University, Key Laboratory of Environmental Biology and Pollution Control (Hunan University), Ministry of Education, and School of Chemical and Biomedical Engineering, Nanyang Technological University developed Fe-based MOFs (Fe-MIL-101, Fe-MIL-100, Fe-MIL-53) with characteristics such as specific surface areas, pore volumes, pore sizes, and visible-light response, achieving high efficiency in the adsorption and photocatalytic degradation of tetracycline in the field of environmental pollutant treatment.
Background:
1. Tetracycline, a widely used antibiotic, causes environmental pollution and threats to human health and ecological balance. Previous researchers tested methods like adsorption, biological degradation, and photocatalytic degradation for tetracycline removal, with photocatalytic degradation being favorable but still needing efficient catalysts.
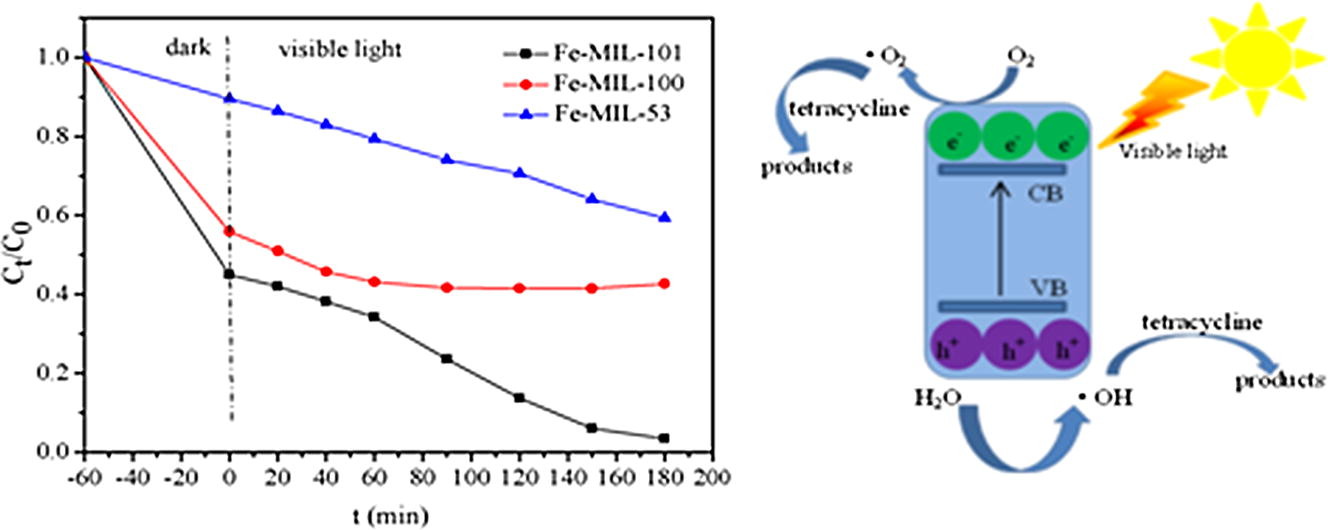
2. The authors compared three Fe-based MOFs (Fe-MIL-101, Fe-MIL-100, Fe-MIL-53) in tetracycline removal, finding Fe-MIL-101 had the best performance, with 96.6% removal of 50 mg/L tetracycline.
Research Content:
1. Synthesis:
The authors synthesized Fe-MIL-101, Fe-MIL-100, and Fe-MIL-53 using hydrothermal methods with modifications. For Fe-MIL-101, FeCl₃·6H₂O and H₂BDC were dissolved in DMF, heated at 110°C for 20 h, purified, and activated. Fe-MIL-53 was synthesized with equal molar FeCl₃·6H₂O and H₂BDC in DMF, heated at 150°C for 12 h. Fe-MIL-100 used Fe(NO₃)₃·9H₂O, H₃BTC, and hydrofluoric acid, heated at 160°C for 12 h.
2. Characterizations:
1) BET results: Fe-MIL-101 had a surface area of 252.59 m²/g, pore volume 0.86 cm³/g, pore size 25.74 nm; Fe-MIL-100: 1203.36 m²/g, 0.34 cm³/g, 2.27 nm; Fe-MIL-53: 21.42 m²/g, 0.04 cm³/g, 4.60 nm.
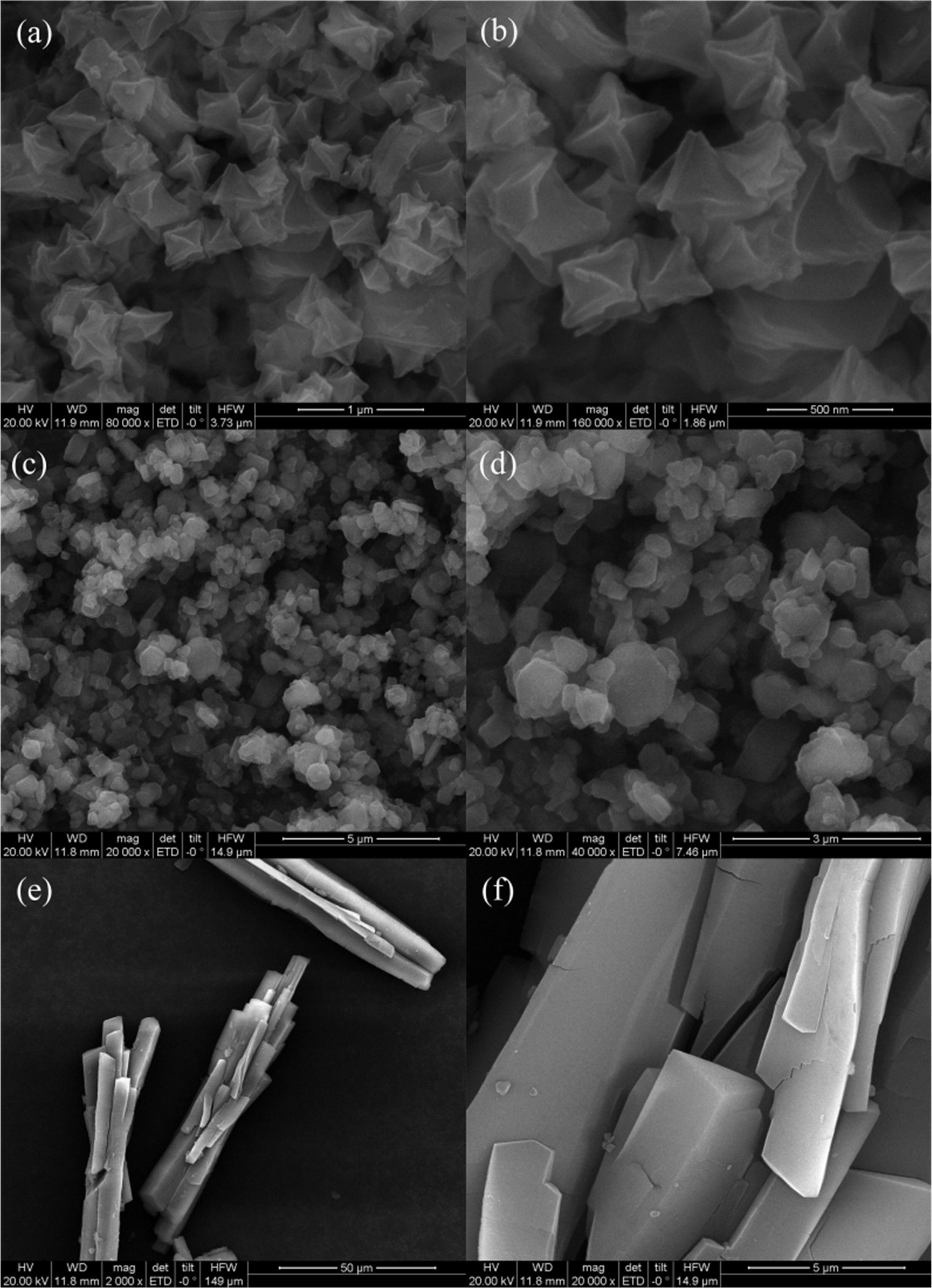
2) SEM tests: Fe-MIL-101 particles were regular octahedrons with uniform size (~500 nm); Fe-MIL-100 had granular particles (500 nm–1 μm); Fe-MIL-53 resembled bundled sticks (diameter ~80 μm).
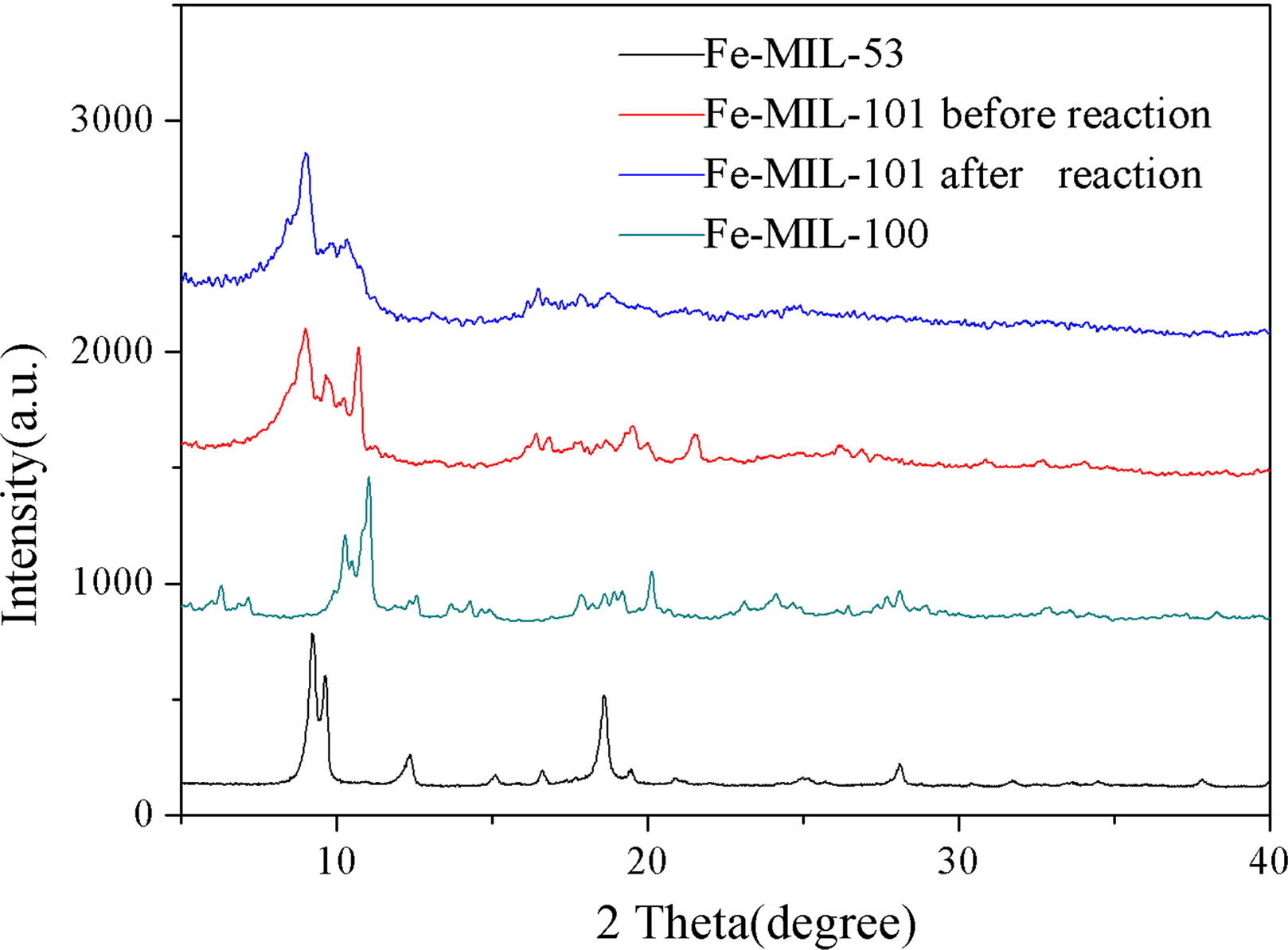
3) Other tests: XRD confirmed pure phases; XPS showed Fe-MIL-101 contained Fe, C, O; UV–vis diffuse reflectance spectra gave band gaps: Fe-MIL-101 (1.88 eV), Fe-MIL-100 (2.06 eV), Fe-MIL-53 (1.97 eV).
3. Application:
The materials were tested for tetracycline adsorption and photocatalytic degradation. Fe-MIL-101 had the highest adsorption (55.1% at equilibrium) and photocatalytic removal (96.6% after 3 h visible light). Optimum Fe-MIL-101 dosage was 0.5 g/L; removal efficiency decreased with initial tetracycline concentration. It showed good recyclability.
4. Mechanism:
Adsorption kinetics fitted pseudo-second-order model (chemical adsorption dominant). Fe-MIL-101 adsorption followed Freundlich model (heterogeneous sites); others followed Langmuir (monolayer). Photocatalysis involved O₂⁻, ·OH, and h⁺ as active species (trapping and ESR tests). Fe-MIL-101's excellent performance was due to large surface area, pore volume, strong visible light absorption, and efficient radical generation.
Outlook:
This research demonstrates Fe-based MOFs, especially Fe-MIL-101, as promising for tetracycline and antibiotic degradation, expanding MOFs' environmental applications with high efficiency and recyclability.
Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs
Authors: Dongbo Wang, Feiyue Jia, Hou Wang, Fei Chen, Ying Fang, Wenbo Dong, Guangming Zeng, Xiaoming Li, Qi Yang, Xingzhong Yuan
DOI: 10.1016/j.jcis.2018.02.067
Link: https://www.sciencedirect.com/science/article/pii/S0021979718302224
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.


