Home >
News > Fe-based MOFs for efficient adsorption and degradation of acid orange 7 in aqueous solution via persulfate activation
Fe-based MOFs for efficient adsorption and degradation of acid orange 7 in aqueous solution via persulfate activation
Summary:
The authors from School of Resources and Environment, University of Jinan developed Fe-based metal–organic frameworks (MIL-101(Fe), MIL-100(Fe), MIL-53(Fe), MIL-88B(Fe)) with high adsorption capacity and catalytic activity, achieving efficient removal of acid orange 7 (AO7) in aqueous solution via persulfate activation in the field of environmental pollution treatment.
Background:
1. Sulfate radicals-based advanced oxidation processes (SR-AOPs) are effective for degrading recalcitrant organic pollutants, but developing efficient heterogeneous catalysts for persulfate activation is crucial. Metal–organic frameworks (MOFs) have shown potential in adsorption and catalysis, but Fe-based MOFs' performance in AO7 removal via persulfate activation needs comparison and study.
2. The authors prepared four Fe-based MILs via solvothermal method, compared their adsorption and catalytic degradation abilities for AO7 in the presence of persulfate, and explored the mechanism, obtaining significant results.
Research Content:
1. Synthesis:
The authors synthesized MIL-101(Fe), MIL-100(Fe), MIL-53(Fe), and MIL-88B(Fe) using a solvothermal method with FeCl₃·6H₂O and corresponding ligands (e.g., BDC for MIL-101(Fe)).
2. Characterizations:
1) BET results showed the specific surface areas were 2986 m²/g (MIL-101(Fe)), 1798 m²/g (MIL-100(Fe)), 965 m²/g (MIL-53(Fe)), and 19.2 m²/g (MIL-88B(Fe)). Pore sizes varied, with MIL-101(Fe) having cages of 29 Å and 34 Å.
2) SEM tests showed MIL-101(Fe) had octahedron morphology with average diameter 800 nm; MIL-100(Fe) had poor crystallization; MIL-53(Fe) was sphere-like (200–800 nm); MIL-88B(Fe) was spindle-shaped (0.75 μm in length, 0.3 μm in diameter).
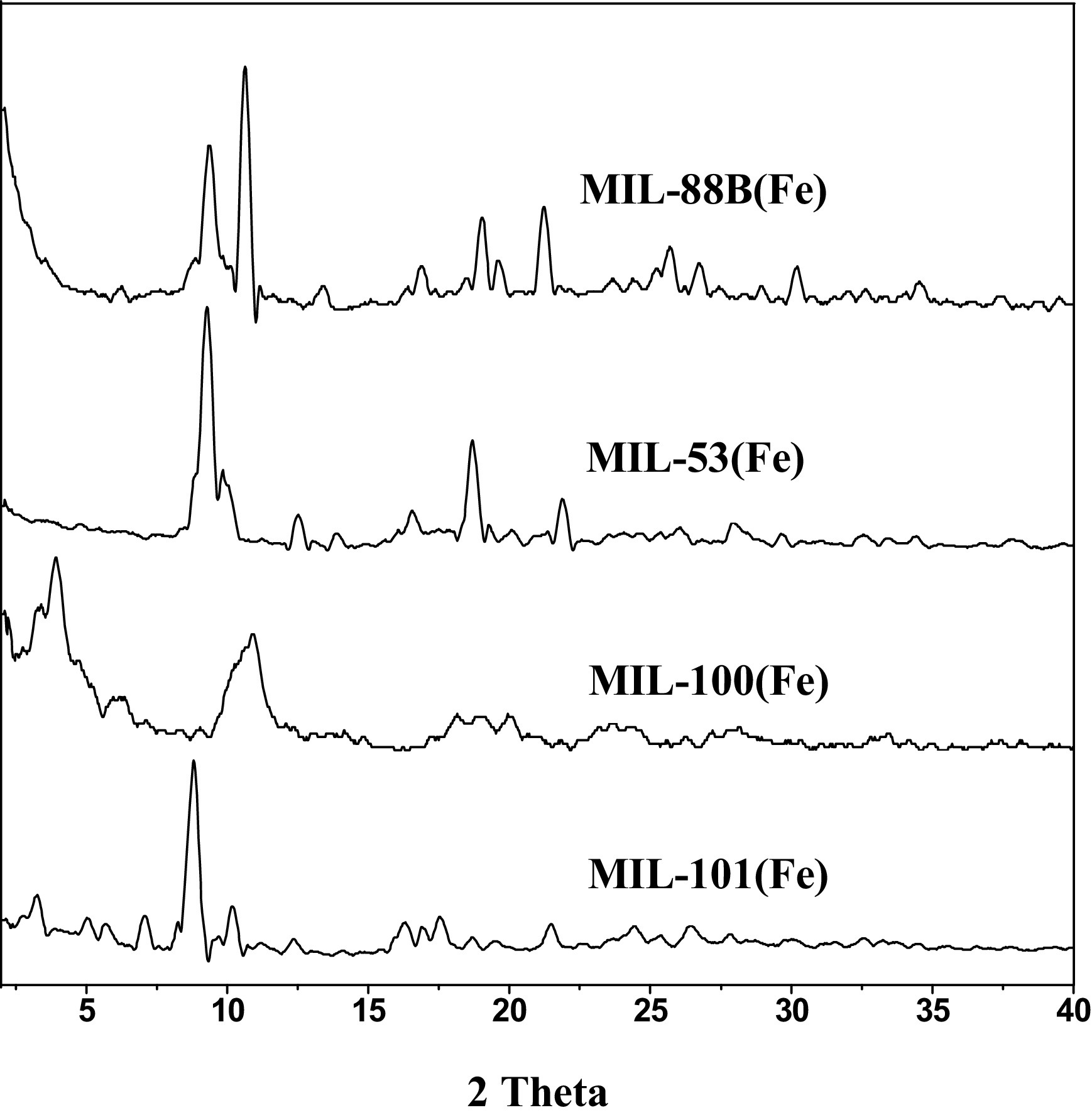
3) XRD confirmed the crystal structures; FTIR showed characteristic peaks of carboxylate groups; XPS indicated Fe³⁺ in MILs, with some converting to Fe²⁺ after reaction.
3. Application:
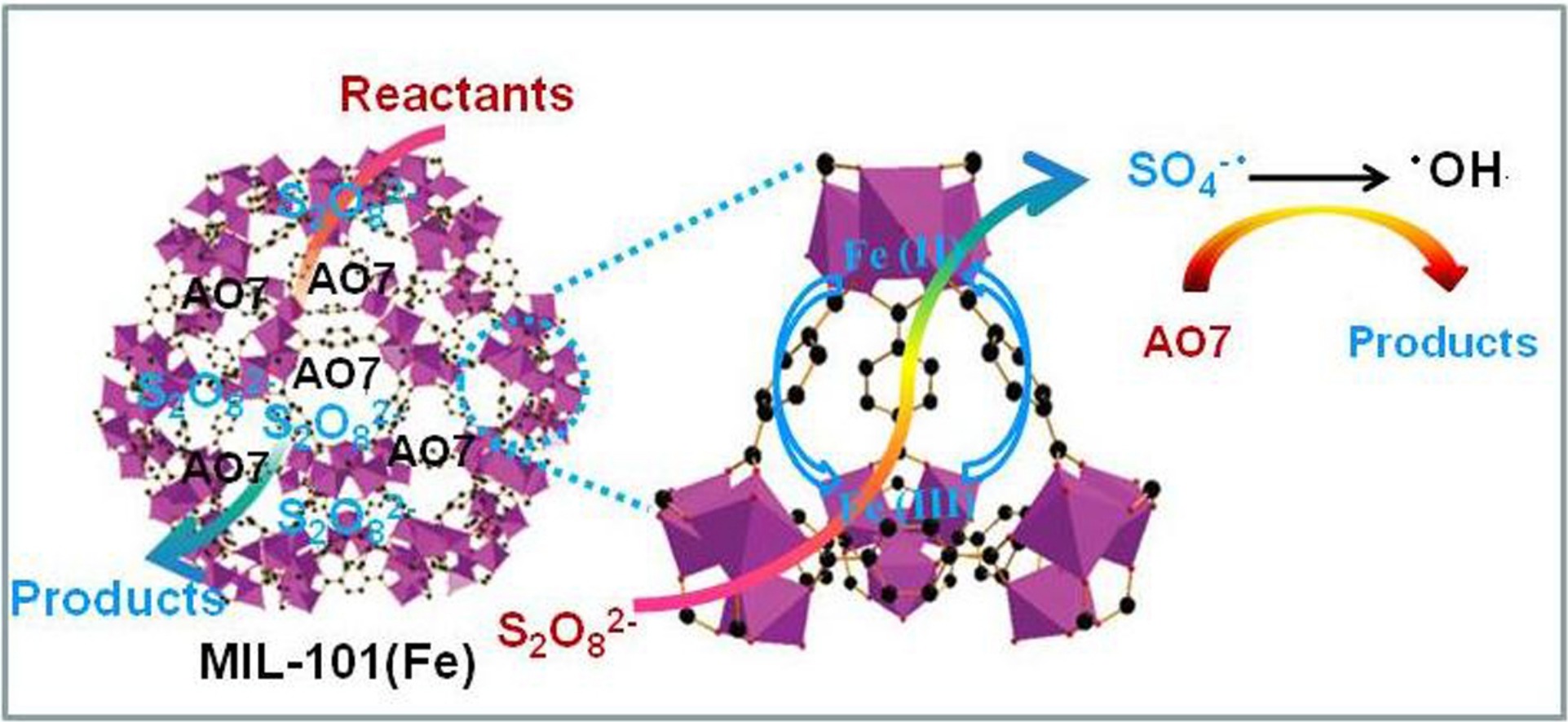
The materials were tested for AO7 removal. Adsorption capacities followed MIL-101(Fe) > MIL-100(Fe) > MIL-53(Fe) > MIL-88B(Fe), with MIL-101(Fe) reaching 153.4 mg/g. In catalytic degradation with persulfate, the order was the same, with MIL-101(Fe) having the highest rate constant (0.017 min⁻¹). All showed good reusability over three cycles.
4. Mechanism:
The materials adsorbed AO7 first. Then, Fe³⁺ in MILs activated persulfate to generate SO₄⁻· and ·OH via redox reactions (Fe³⁺ ↔ Fe²⁺). These radicals degraded AO7. The high surface area and active Fe sites contributed to the performance.
Outlook:
This research clarifies the performance differences of Fe-based MILs in AO7 removal, demonstrating their potential as efficient catalysts for environmental pollution control, providing a basis for practical applications.
Fe-based MOFs for efficient adsorption and degradation of acid orange 7 in aqueous solution via persulfate activation
Authors: Xianghui Li, Weilin Guo, Zhonghua Liu, Ruiqin Wang, Hua Liu
DOI: 10.1016/j.apsusc.2016.02.037
Link: https://www.sciencedirect.com/science/article/pii/S0169433216303277
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

