Home >
News > Selective recognition of argon by an aluminum-based MOF for oxygen recovery from Ar/O₂ mixture under ambient conditions
Selective recognition of argon by an aluminum-based MOF for oxygen recovery from Ar/O₂ mixture under ambient conditions
Summary:
The authors from South China University of Technology and Foshan University developed aluminum-based MOFs (Al-BDC and Al-FUM) with tunable pore structures and high stability, among which Al-FUM achieves selective adsorption of Ar, enabling one-step production of high-purity O₂ (>99.99%) from Ar/O₂ mixtures in the field of oxygen recovery.
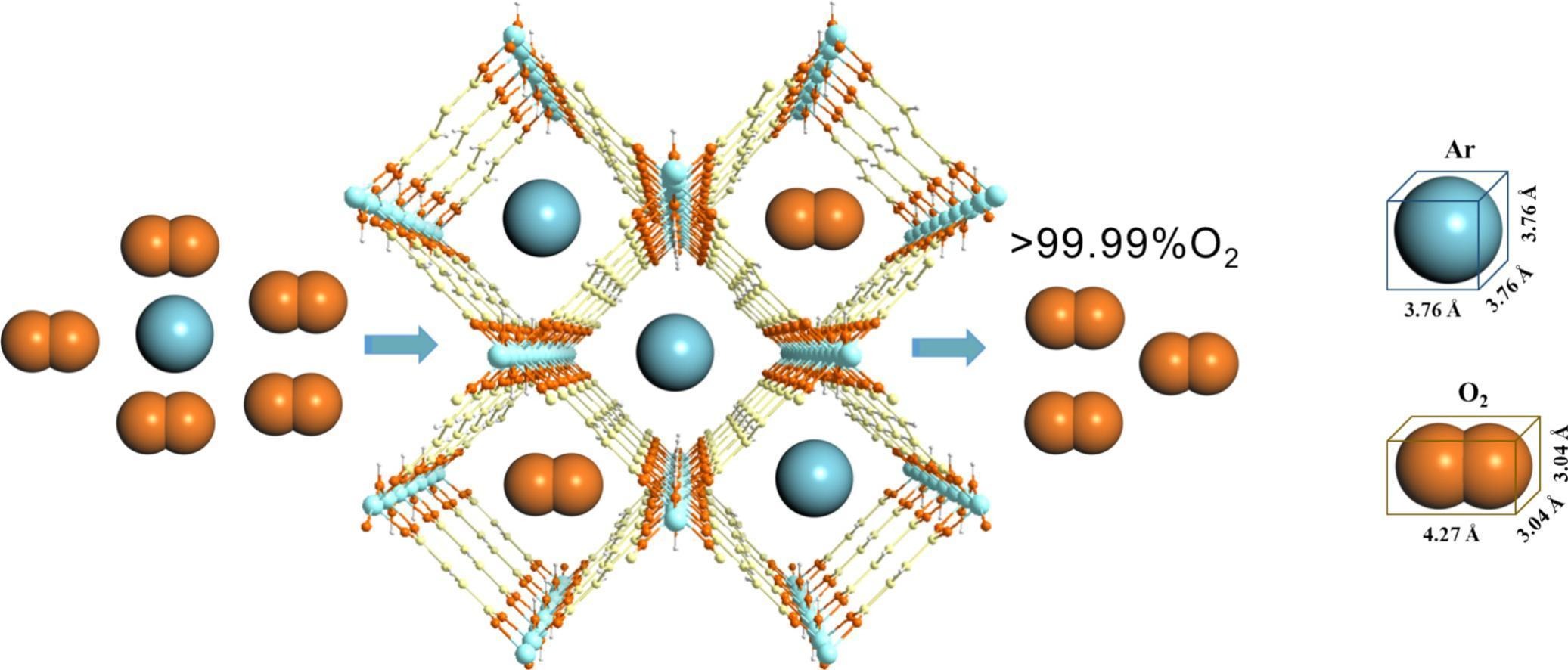
Background:
1. To address the problem of low purity (only 95%) in oxygen produced by pressure swing adsorption (PSA) due to difficult separation of Ar and O₂, previous researchers developed Ar-selective adsorbents such as Ag-exchanged zeolites (e.g., AgA, AgZSM-5) with moderate performance but high cost and poor stability; non-silver materials (e.g., Mo/NaY) show insufficient selectivity and adsorption capacity.
2. The authors proposed an innovative method by optimizing ligand length and regulating pore polarity, synthesizing Al-FUM with reduced pore size and enhanced surface polarity, which exhibits excellent Ar selectivity and stability, overcoming the limitations of existing materials.
Research Content:
1. Synthesis:
- Al-BDC was synthesized via solvothermal method: Al(NO₃)₃·9H₂O and terephthalic acid (H₂BDC) were reacted in water at 220°C for 72 hours, followed by DMF solvent exchange and calcination at 280°C.
- Al-FUM was synthesized via solvothermal method: AlCl₃·6H₂O and fumaric acid (H₂FUM) were reacted in DMF at 130°C for 96 hours, followed by acetone solvent exchange and vacuum drying at 130°C.
2. Characterizations:
1) BET and pore size distribution: Al-BDC has a BET surface area of 1472 m²/g, pore size of 10.91 Å; Al-FUM has a BET surface area of 1084 m²/g, pore size of 5.90 Å (calculated by DFT).
2) SEM/TEM tests show the particle size of the material: Both MOFs exhibit typical microporous morphologies, with no specific particle size data provided, but PXRD confirms their crystalline structures.
3) Other tests: PXRD verifies phase purity; TGA shows Al-BDC and Al-FUM are stable up to 560°C and 430°C, respectively; Al-FUM maintains structural integrity after immersion in various solvents (e.g., water, ethanol) and exposure to 75% RH for 12 days, demonstrating excellent chemical stability.
3. Application:
The materials were tested in Ar/O₂ separation:
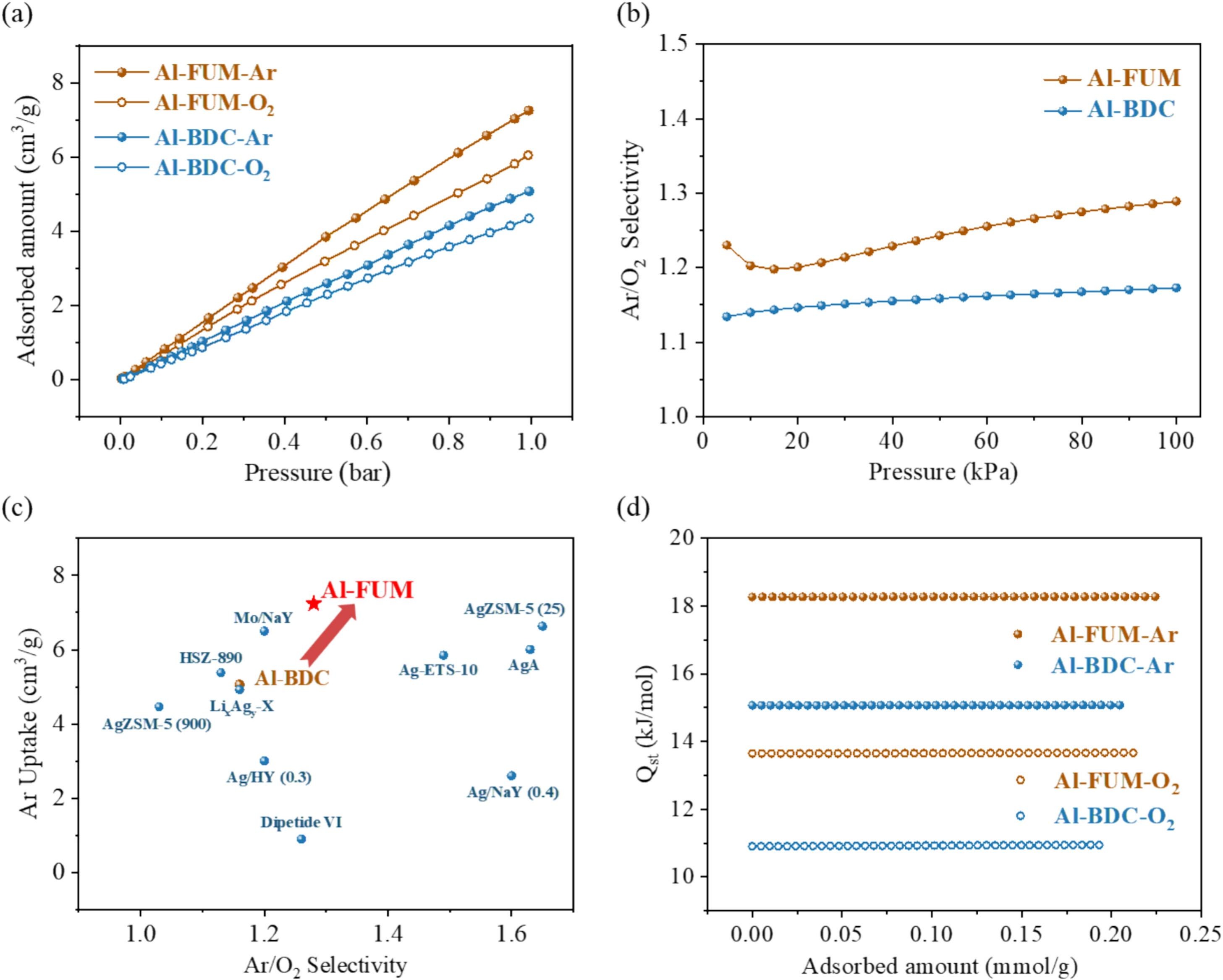
- Static adsorption: Al-FUM adsorbs 7.24 cm³/g Ar and 6.02 cm³/g O₂ at 298 K/1 bar, with Ar/O₂ selectivity of 1.28 (IAST).
- Dynamic breakthrough: Al-FUM separates Ar/O₂ (5:95) mixture at 298 K/1 bar, producing 99.99% O₂ with a productivity of 4.72 L/kg, and maintains performance over 3 cycles.
4. Mechanism:
- Al-FUM has a smaller pore size (5.90 Å) matching Ar's kinetic diameter, enhancing confinement effects and van der Waals interactions with Ar.
- The higher molecular polarity index (MPI) of H₂FUM ligand in Al-FUM strengthens electrostatic interactions with Ar (higher polarizability than O₂), promoting preferential Ar adsorption.
- Grand Canonical Monte Carlo simulations confirm Ar interacts more strongly with μ-OH groups and ligand H atoms in Al-FUM, with interaction energy difference (2.24 kJ/mol) larger than that in Al-BDC (1.40 kJ/mol).
Outlook:
This research develops a stable, low-cost Al-FUM with superior Ar-selective adsorption, realizing one-step production of high-purity oxygen from Ar/O₂ mixtures. It provides a new strategy for designing Ar-selective adsorbents and promotes the industrial application of MOFs in gas separation.
Selective recognition of argon by an aluminum-based MOF for oxygen recovery from Ar/O₂ mixture under ambient conditions
Authors: Siyao Zhao, Danxia Lin, Jinze Yao, Xingyan Zhu, Zewei Liu, Zhong Li, Qibin Xia
DOI: 10.1016/j.ces.2025.122130
Link: https://www.sciencedirect.com/science/article/pii/S0009250925009534
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

