Home >
News > Carbonized Nanoscale Metal-Organic Frameworks as High Performance Electrocatalyst for Oxygen Reduction Reaction
Carbonized Nanoscale Metal-Organic Frameworks as High Performance Electrocatalyst for Oxygen Reduction Reaction
Summary:
The authors from Harbin Institute of Technology, National Center for Nanoscience and Technology, and Griffith University developed carbonized nanoparticles (CNPs) derived from nanoscale metal-organic frameworks (MIL-88B-NH₃) with high catalytic activity, good stability, and excellent methanol tolerance, achieving superior results in the application of oxygen reduction reaction (ORR) and alkaline direct methanol fuel cells (ADMFCs).
Background:
1. To address the issues of high cost, scarcity, and poor stability of Pt-based electrocatalysts for ORR, previous researchers developed non-noble metal catalysts via pyrolysis of organic-inorganic hybrids. However, these catalysts generally showed low activity due to uncontrollable precursor size/shape and insufficient exposure of active sites.
2. The authors proposed an innovative method using nanoscale MIL-88B-NH₃ as precursors for pyrolysis, successfully preparing CNPs with ORR activity surpassing commercial Pt/C and excellent performance in ADMFCs.
Research Content:
1. Synthesis:
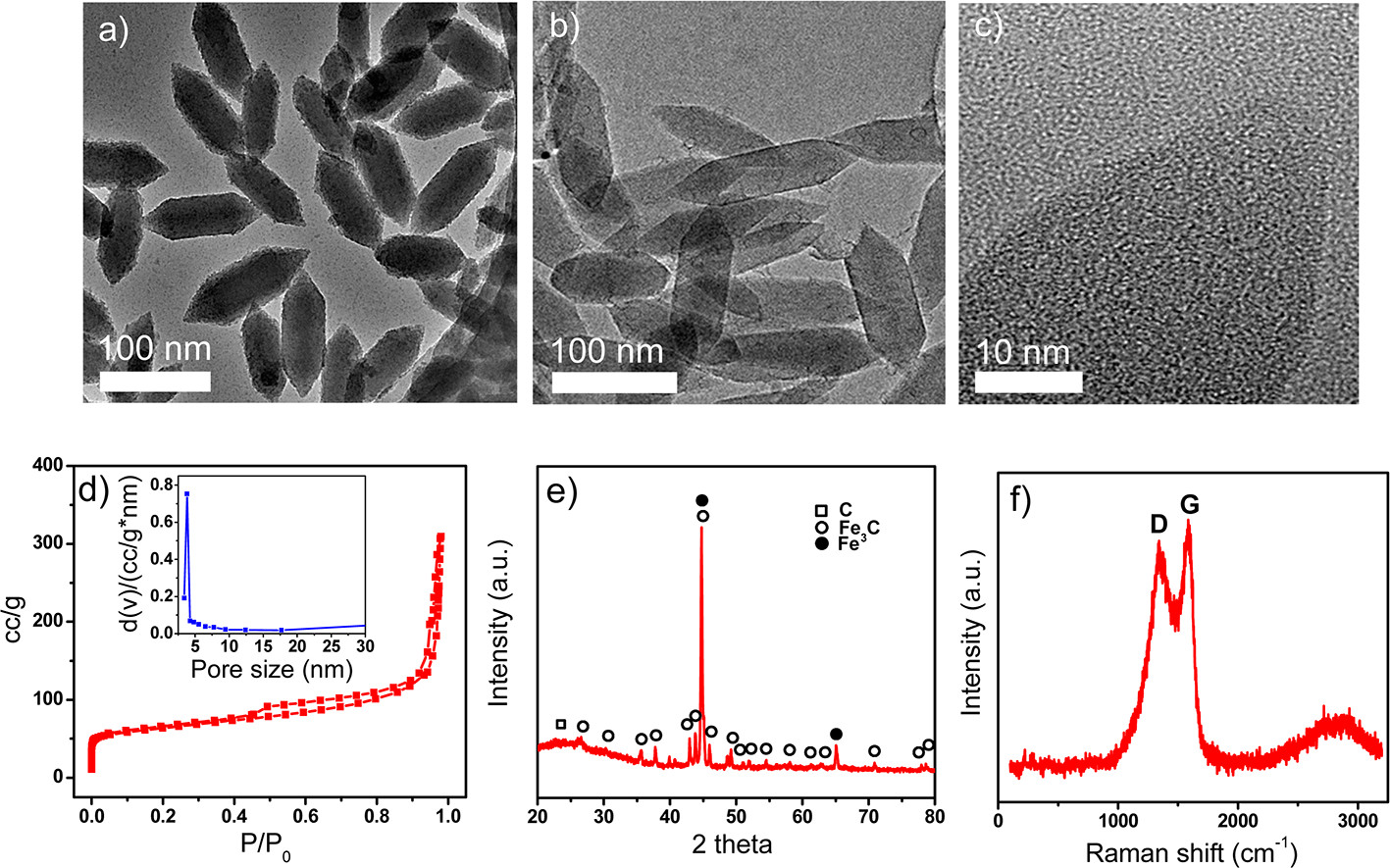
The authors synthesized MIL-88B-NH₃ nanoparticles (spindle-like, ~50 nm in diameter, ~140 nm in length) using FeCl₃·6H₂O and 2-aminoterephthalic acid with Pluronic®F127 as a capping agent via hydrothermal method. CNPs were obtained by pyrolyzing these nanoparticles at 900°C in Ar atmosphere for 6 hours.
2. Characterizations:
1) BET and pore size distribution: CNPs have a BET surface area of 326 m²/g, a uniform pore size of 3.8 nm, and a total pore volume of 0.679 cm³/g.
2) SEM/TEM tests show the particle size of the material: CNPs retain the spindle-like shape of MIL-88B-NH₃ nanoparticles with similar size (~50 nm in diameter, ~140 nm in length); in contrast, carbonized microparticles (CMPs) derived from microscale MIL-88B-NH₃ show collapsed structures.
3) Other tests: XPS confirms CNPs contain C, N, O, and Fe, with pyridine-type and quaternary-type nitrogen; XRD reveals the presence of Fe, Fe₃C, and amorphous carbon; Raman spectroscopy indicates high disorder of carbon with significant edge plane sites.
3. Application:
The material was tested in ORR and ADMFCs:
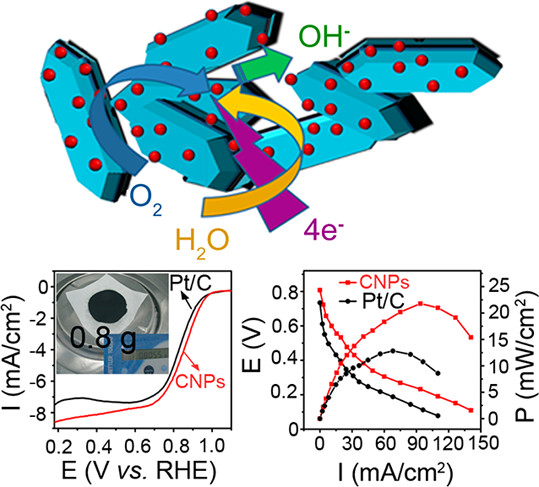
- In ORR (0.1 M KOH), CNPs exhibit an onset potential of 1.03 V (vs RHE), a half-wave potential of 0.92 V, and a diffusion-limited current density of 8.31 mA/cm², outperforming Pt/C.
- In ADMFCs at 60°C, the maximum power density reaches 22.7 mW/cm², 1.7 times that of Pt/C.
- CNPs show better stability (21% current loss after 40000 s) and methanol tolerance than Pt/C.
4. Mechanism:
The high ORR performance of CNPs is attributed to:
- Nanoscale size preserving porous structure, facilitating mass transport;
- Fe/Fe₃C as active sites, and pyridine-type/quaternary-type nitrogen enhancing catalytic activity;
- High sp² carbon content improving conductivity;
- A dominant 4-electron transfer pathway (n≈3.98) ensuring high efficiency.
Outlook:
This research realizes the preparation of high-performance non-noble metal ORR catalysts via pyrolysis of nanoscale MOFs, which not only surpasses commercial Pt/C in activity, stability, and cost-effectiveness but also provides a feasible strategy for the rational design of carbon-based catalysts, promoting the practical application of fuel cells and other clean energy technologies.
Carbonized Nanoscale Metal-Organic Frameworks as High Performance Electrocatalyst for Oxygen Reduction Reaction
Authors: Shenlong Zhao, Huajie Yin, Lei Du, Liangcan He, Kun Zhao, Lin Chang, Geping Yin, Huijun Zhao, Shaoqin Liu, Zhiyong Tang
DOI: 10.1021/nn505582e
Link: https://pubs.acs.org/doi/10.1021/nn505582e
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

