Home >
News > Sustainable Preparation of MIL-100(Fe) and Its Photocatalytic Behavior in the Degradation of Methyl Orange in Water
Sustainable Preparation of MIL-100(Fe) and Its Photocatalytic Behavior in the Degradation of Methyl Orange in Water
Summary:
The authors from Aksum University, Instituto de Catálisis y Petroleoquímica (ICP-CSIC), and Laboratorio de Microscopías Avanzadas (LMA), Instituto de Nanociencia de Aragon (INA), Universidad de Zaragoza developed MIL-100(Fe) with high crystallinity, large specific surface area, and suitable band gap, achieving good results in the application of photocatalytic degradation of methyl orange in water.
Background:
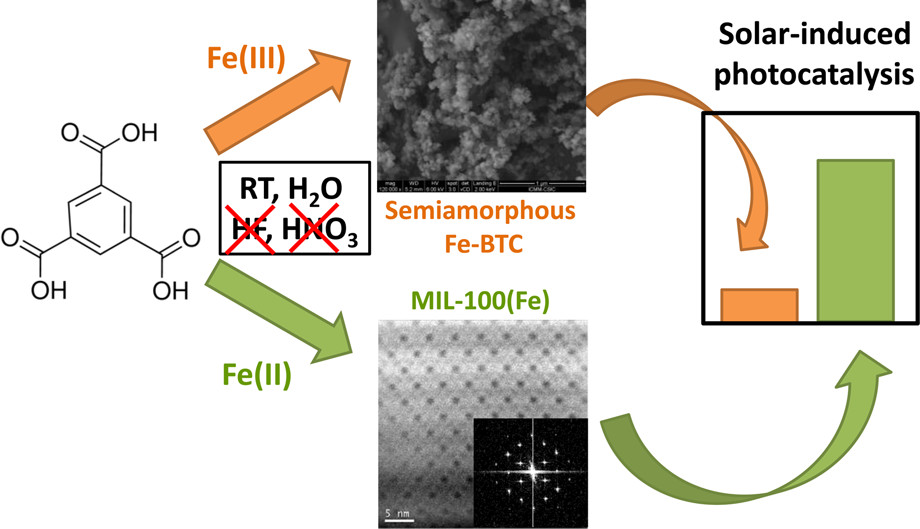
1. The conventional synthesis of MIL-100(Fe) requires high temperature (150 °C), long time (6 days), uses corrosive acids (HF, HNO₃), and needs high-temperature washing, which is unsustainable. Previous studies tried to improve but failed to achieve full sustainability.
2. The authors proposed a sustainable synthesis method for MIL-100(Fe) at room temperature, with short time, no corrosive acids, and room-temperature washing, obtaining high-quality materials.
Research Content:
1. Synthesis:
The authors synthesized MIL-100(Fe)-RT using FeCl₂·4H₂O (Fe²⁺ source) and H₃BTC with NaOH in water, reacting at room temperature for 24 h; Fe-BTC-RT was synthesized using FeCl₃·6H₂O (Fe³⁺ source) under similar conditions.
2. Characterizations:
1) BET: MIL-100(Fe)-RT has BET surface area 1974 m²/g, pore volume 0.75 cm³/g, with pore size peaks at 1.85 and 2.26 nm; Fe-BTC-RT has BET 925 m²/g, pore volume 0.65 cm³/g, and one pore peak at 1.85 nm.
2) SEM/TEM tests show MIL-100(Fe)-RT has faceted particles (500 nm - 1 μm) with hexagonal pore arrays and Fd-3m symmetry (unit cell 70.74 Å); Fe-BTC-RT is mostly amorphous, with a few crystals (unit cell 67.76 Å).
3) DR-UV-vis shows band gaps: 3.08 eV (MIL-100(Fe)-RT), 3.19 eV (Fe-BTC-RT); TGA shows similar decomposition to conventional MIL-100(Fe).
3. Application:
MIL-100(Fe)-RT degrades 64% of 5 ppm methyl orange under UV light (7 h) and 40% under sunlight; Fe-BTC-RT has negligible activity.
4. Mechanism:
MIL-100(Fe)-RT has larger cages (29 Å) allowing methyl orange (16.7×6.1×5.2 Å) access to active sites; Fe-BTC-RT only has smaller cages, restricting access.
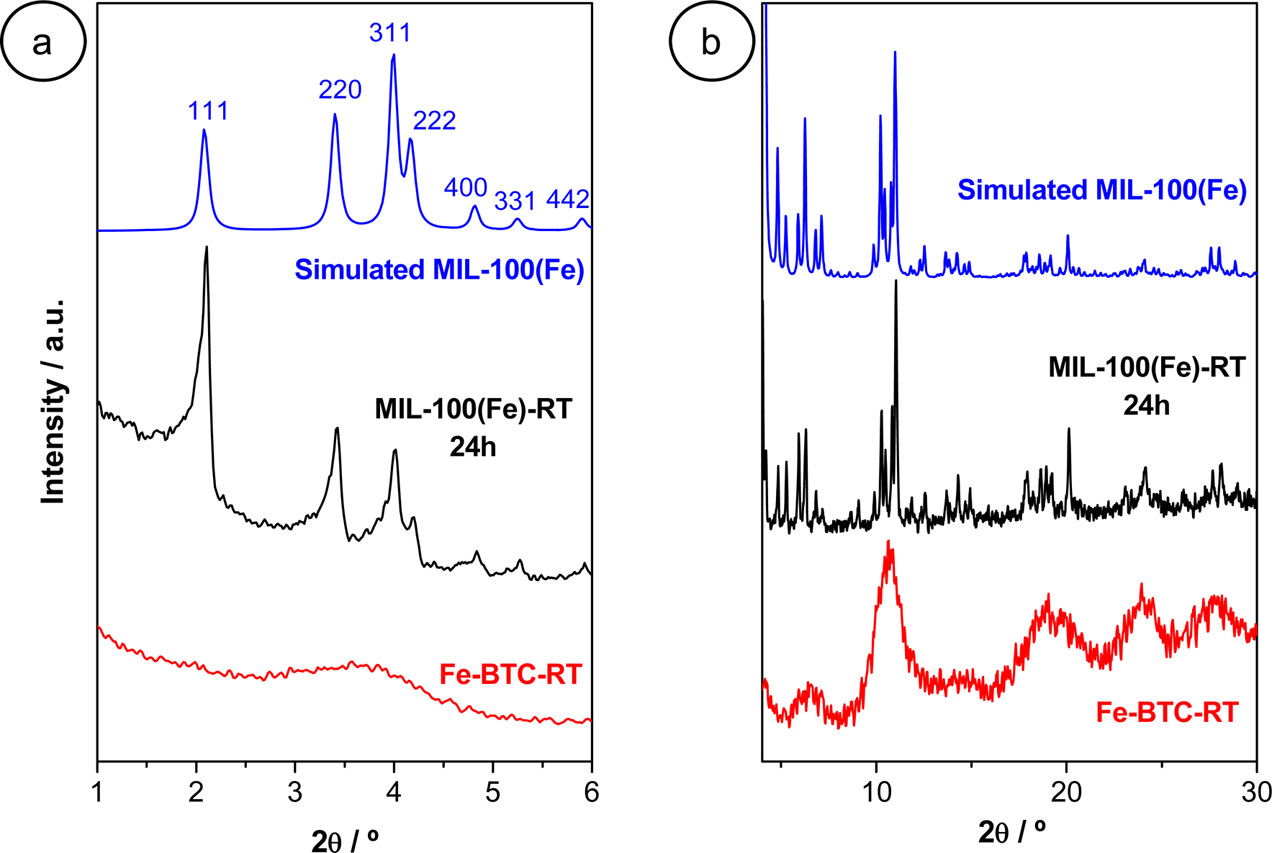
Outlook:
This research achieves sustainable synthesis of high-quality MIL-100(Fe), with good photocatalytic performance, contributing to MOF sustainability and water treatment applications.
Sustainable Preparation of MIL-100(Fe) and Its Photocatalytic Behavior in the Degradation of Methyl Orange in Water
Authors: Kiros Guesh, Clarice A. D. Caiuby, Álvaro Mayoral, Manuel Díaz-García, Isabel Díaz, Manuel Sánchez-Sánchez
DOI: 10.1021/acs.cgd.6b01776
Link: https://pubs.acs.org/doi/10.1021/acs.cgd.6b01776
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

