Home >
News > Iron metal-organic framework as highly efficient catalyst for ozone decomposition
Iron metal-organic framework as highly efficient catalyst for ozone decomposition
Summary:
The authors fromBeijing Institute of Technology (China) andResearch Institute of Petroleum Exploration & Development, China National Petroleum Corporation (China) developed an iron metal-organic framework MIL-100(Fe), which has high specific surface area, water stability, and uniformly distributed Fe active sites. It achieved 100% ozone conversion efficiency lasting over 100 h under ambient conditions in the application ofozone decomposition and air filtration.
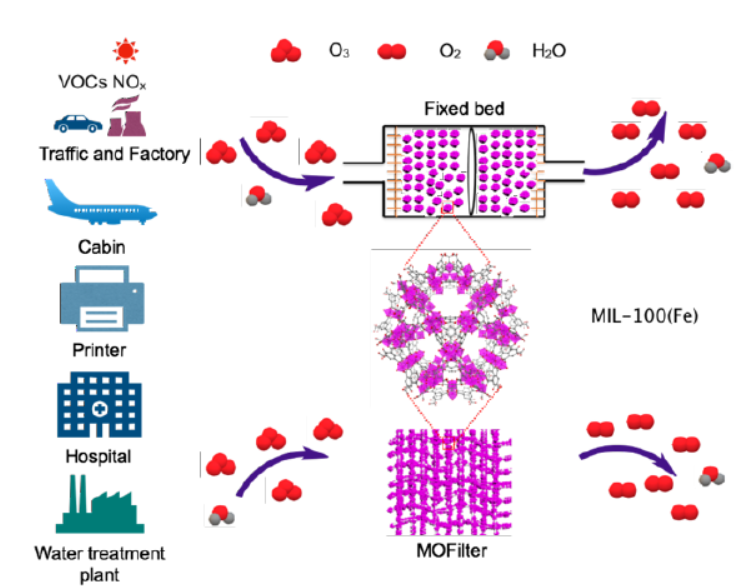
Background:
1. To address the problem ofozone pollution (hazardous to health, widespread in both outdoor and confined spaces) and the limitations of existing ozone removal methods:
- Activated carbon adsorption requires sorbent regeneration and produces waste.
- Transition metal oxides (e.g., α-MnO₂) have drastically reduced activity due to surface oxygen accumulation and water vapor competitive adsorption.
- Noble metal catalysts are costly and scarce, limiting large-scale use.
2. The authors proposed using the iron metal-organic frameworkMIL-100(Fe) as an ozone decomposition catalyst, which exhibits high efficiency, long-term stability under high humidity, and processability into films—filling the gap of non-noble metal catalysts for humid environment applications.
Research Content:
1. Synthesis
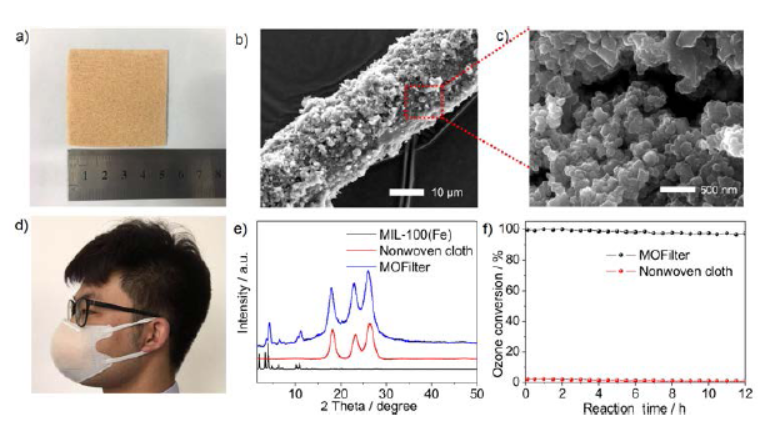
The authors synthesized nanosized MIL-100(Fe) usingFeCl₃·6H₂O (iron source) andtrimesic acid (organic ligand) via a previously reported method, followed by activation at 150 °C for 12 h to remove residual solvent molecules.
2. Characterizations
1.BET and pore size distribution: N₂ sorption experiments showed MIL-100(Fe) has a Brunauer-Emmett-Teller (BET) specific surface area of 1726 m²/g and an accessible pore diameter of 1.68 nm, enabling free diffusion and selective adsorption of ozone molecules.
2.SEM/XRD tests: SEM confirmed no morphological change after 100 h of ozone exposure; XRD patterns of as-synthesized and ozone-exposed MIL-100(Fe) matched the simulated one, proving high crystallinity and structural stability.
3.Other tests: X-ray photoelectron spectroscopy (XPS) and infrared spectra (IR) further verified the material’s structural stability; differential pressure gauge tests showed the MIL-100(Fe)-based filter (MOFilter) has a low pressure drop of 20 Pa.
3. Application
1.Ozone decomposition performance: At room temperature, 45% relative humidity (RH), and 1.9×10⁵ h⁻¹ space velocity, MIL-100(Fe) maintained 100% ozone conversion for over 100 h—outperforming α-MnO₂ (60% conversion after 12 h) and activated carbon (18% conversion after 12 h).
2.Humidity adaptability: It achieved complete ozone removal at 40%–>90% RH; even at <5% RH, switching back to 40% RH restored 100% conversion.
3.Practical filtration: MIL-100(Fe) was processed into MOFilter via hot-pressing onto non-woven cloth. As a mask filtration layer, it completely decomposed 200 ppb ozone for 12 h with low pressure drop.
4. Mechanism
1.Experimental result analysis: Water plays a synergistic role (not competitive inhibition) by promoting the formation and decomposition of peroxide/higher valent oxide intermediates on MIL-100(Fe) surface sites.
2.DFT calculation reasoning: Unrestricted Density Functional Theory (UDFT) with B3LYP hybrid functional showed:
- In moist conditions, Fe(III)-bound water in MIL-100(Fe) deprotonates to form Fe(III)-OH⁻.
- The rate-limiting step is hydrogen transfer from Fe(III)-OH⁻ to O₃ (barrier: 25.5 kcal/mol), followed by rebound reactions to generate O₂ and regenerate Fe(III) active sites—realizing cyclic catalytic ozone decomposition.
Outlook:
This research is the first to explore MOFs for catalytic ozone decomposition, verifying MIL-100(Fe)’s superiority over traditional catalysts. Its processability into practical filters (e.g., mask layers) provides a new direction for non-noble metal catalyst design and promotes the application of MOFs in air pollution control.
Iron metal-organic framework as highly efficient catalyst for ozone decomposition
Authors: Hang Wang, Pietro Rassu, Xiao Wang, Haiwei Li, Xiaorui Wang, Xiaoqi Wang, Xiao Feng, Anxiang Yin, Pengfei Li, Xu Jin, Shi-Lu Chen, Xiaojie Ma, Bo Wang
DOI: 10.1002/anie.201810268;
Link: https://onlinelibrary.wiley.com/doi/10.1002/anie.201810268
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

