Home >
News > Acid-Resistant Mesoporous Metal–Organic Framework toward Oral Insulin Delivery: Protein Encapsulation, Protection, and Release
Acid-Resistant Mesoporous Metal–Organic Framework toward Oral Insulin Delivery: Protein Encapsulation, Protection, and Release
Summary:
The authors from Northwestern University (USA) and King Abdulaziz University (Saudi Arabia) developed a zirconium-based mesoporous metal-organic framework (MOF) NU-1000 with acid resistance, high porosity, and controlled degradation properties, achieving high insulin loading, effective protection, and targeted release in oral insulin delivery application.
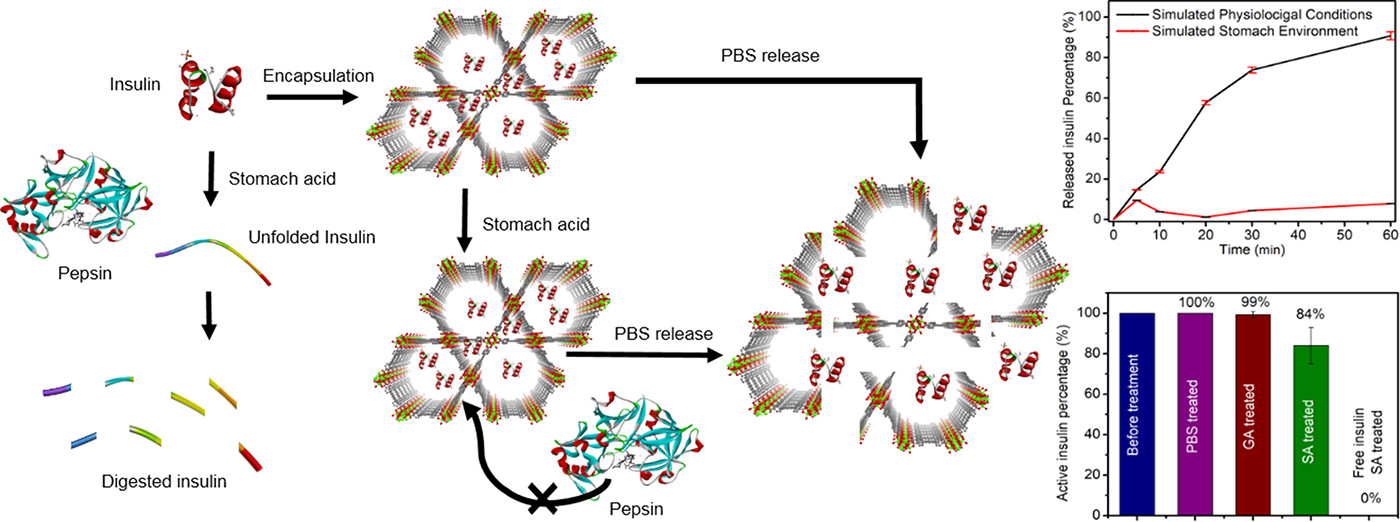
Background:
1. To address the problem of insulin instability in the gastrointestinal tract (degraded by gastric acid/pepsin) and low loading of existing oral insulin carriers, previous researchers used materials like alginate beads, nanoparticles, and collagen as carriers, achieving moderate insulin loading (5-30 wt%), yet there are deficiencies such as requiring excess support materials or harmful adsorption enhancers.
2. The authors in this study proposed using Zr₆-based mesoporous MOF NU-1000 as an insulin carrier, obtaining ~40 wt% insulin loading in 30 min, effective acid protection, and controlled release in physiological environments.
Research Content:
1.Synthesis: The authors synthesized and activated MOF NU-1000 according to a reported procedure; then soaked 2 μm-long NU-1000 crystals in 4×10⁻⁴ g/L insulin aqueous solution (pH 4) at room temperature for 30 min, followed by filtration and washing to prepare insulin@NU-1000 composite.
2.Characterizations:
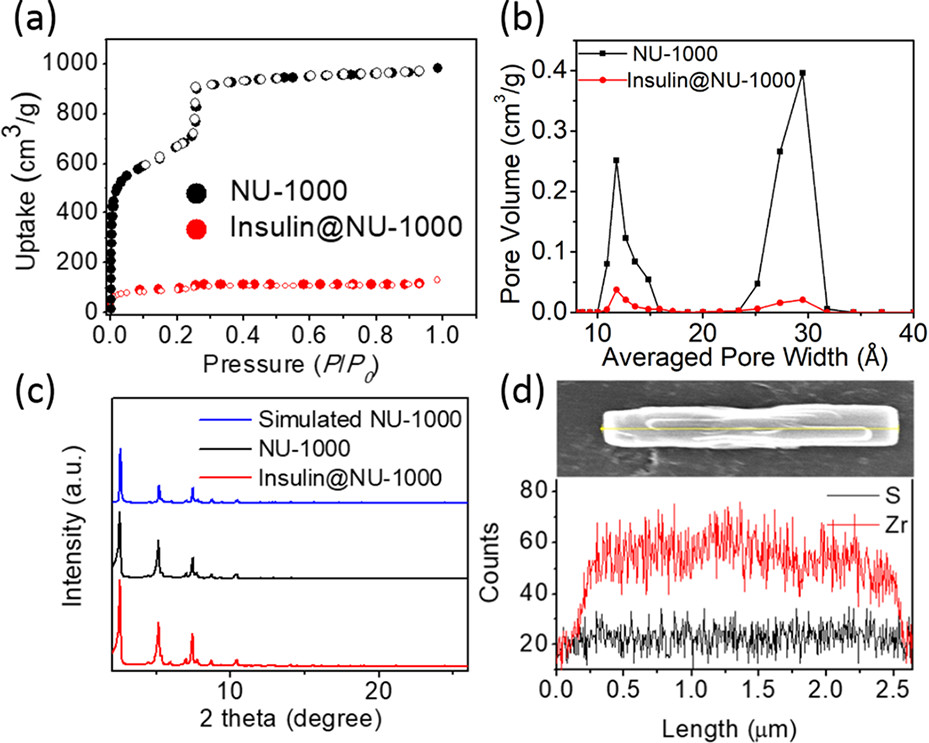
1) BET: N₂ adsorption-desorption showed insulin@NU-1000 had lower N₂ uptake and surface area than pure NU-1000; DFT pore size distribution revealed reduced hexagonal mesopore (~30 Å) and triangular micropore (~12 Å) volumes.
2) SEM tests showed NU-1000 crystals had a length of ~2 μm, and insulin was uniformly distributed in crystals (confirmed by SEM-EDX).
3) Other tests: ICP-OES determined insulin-Zr molar ratio 1.0:7.0, loading ~40 wt%; TGA verified insulin loading; PXRD confirmed NU-1000 retained crystallinity after encapsulation; CLSM observed labeled insulin distribution in NU-1000.
3.Application:
- In simulated gastric acid (pH=1.29): Only 10% insulin released from insulin@NU-1000 after 1 h, and NU-1000 remained stable.
- In simulated physiological condition (pH=7.0): 91% insulin released after 1 h, with 10% NU-1000 linker released; ELISA showed released insulin retained >84% activity.
4.Mechanism:
- NU-1000’s ~30 Å mesopores and ~12 Å micropores allow insulin (13 Å×13 Å) diffusion/encapsulation but exclude pepsin (48 Å×64 Å), avoiding proteolysis.
- Electrostatic/hydrophobic interactions between insulin and NU-1000 promote encapsulation; NU-1000 resists acid (pH≥1) to inhibit insulin unfolding; phosphate in blood cleaves NU-1000 node-linker bonds, triggering insulin release.
Outlook:
This research successfully developed NU-1000 as a high-performance oral insulin carrier, solving key issues of low loading and poor stability of traditional carriers, and providing a feasible solution for clinical oral insulin delivery.
Acid-Resistant Mesoporous Metal–Organic Framework toward Oral Insulin Delivery: Protein Encapsulation, Protection, and Release
Authors: Yijing Chen, Peng Li, Justin A. Modica, Riki J. Drout, Omar K. Farha
DOI: 10.1021/jacs.8b02089
Link: https://pubs.acs.org/doi/10.1021/jacs.8b02089
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

