Home >
News > Node-Accessible Zirconium MOFs
Node-Accessible Zirconium MOFs
Summary:
The authors from Hohai University, Northwestern University, Nanjing University of Science & Technology, and Tianjin University developed node-accessible zirconium MOFs (NU-1000-F, NU-1000-FF-Cl, NU-1000-FF) with controllable formate/Cl content and reversible pore contraction, achieving ~10× higher activity in G-type chemical warfare agent simulant (DMNP) hydrolysis.
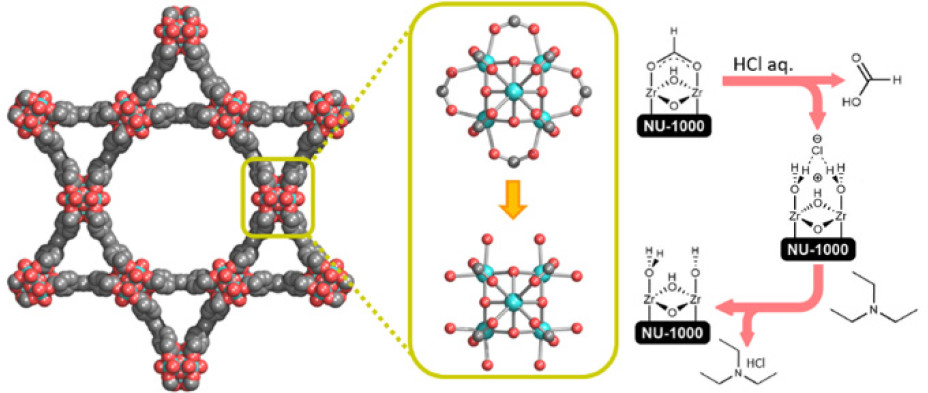
Background:
1. To address the issue that traditional activation of Zr-MOFs (e.g., NU-1000) uses DMF/aq.-HCl, leading to DMF decomposition into formate that occupies Zr₆ node sites (replacing desired OH/H₂O pairs) and reduces active sites, previous researchers used modulators (e.g., benzoate) to synthesize Zr-MOFs but failed to eliminate formate interference, lacking single-crystal evidence for OH/H₂O occupancy.
2. The authors proposed an innovative method: using non-formate-generating solvents (DMSO/NMP/DMA) + aq.-HCl to prepare Cl-containing intermediate NU-1000-FF-Cl, then weak base (TEA/EOA) washing to get formate-free NU-1000-FF, realizing full node accessibility and reversible pore contraction.
Research Content:
1.Synthesis
- as-syn-NU-1000: Powder: ZrOCl₂·8H₂O + benzoic acid in DMF (100℃, 1h) + H₄TBAPy + TFA (100℃, 18h); Single crystal: ZrCl₄ + benzoic acid in DEF (100℃, 1h) + H₄TBAPy + TFA (120℃, 24h).
- NU-1000-F: as-syn-NU-1000 washed with DMF + DMF/8M HCl (100℃, 18h) + acetone exchange + vacuum activation.
- NU-1000-FF-Cl: as-syn-NU-1000 solvent-exchanged with DMSO + DMSO/8M HCl (rt, 18h) + acetone exchange + vacuum activation.
- NU-1000-FF: NU-1000-FF-Cl solvent-exchanged with ethanol + TEA (2 equiv./Zr₆, rt, 18h) + repeated washing + acetone exchange + vacuum activation.
2.Characterizations
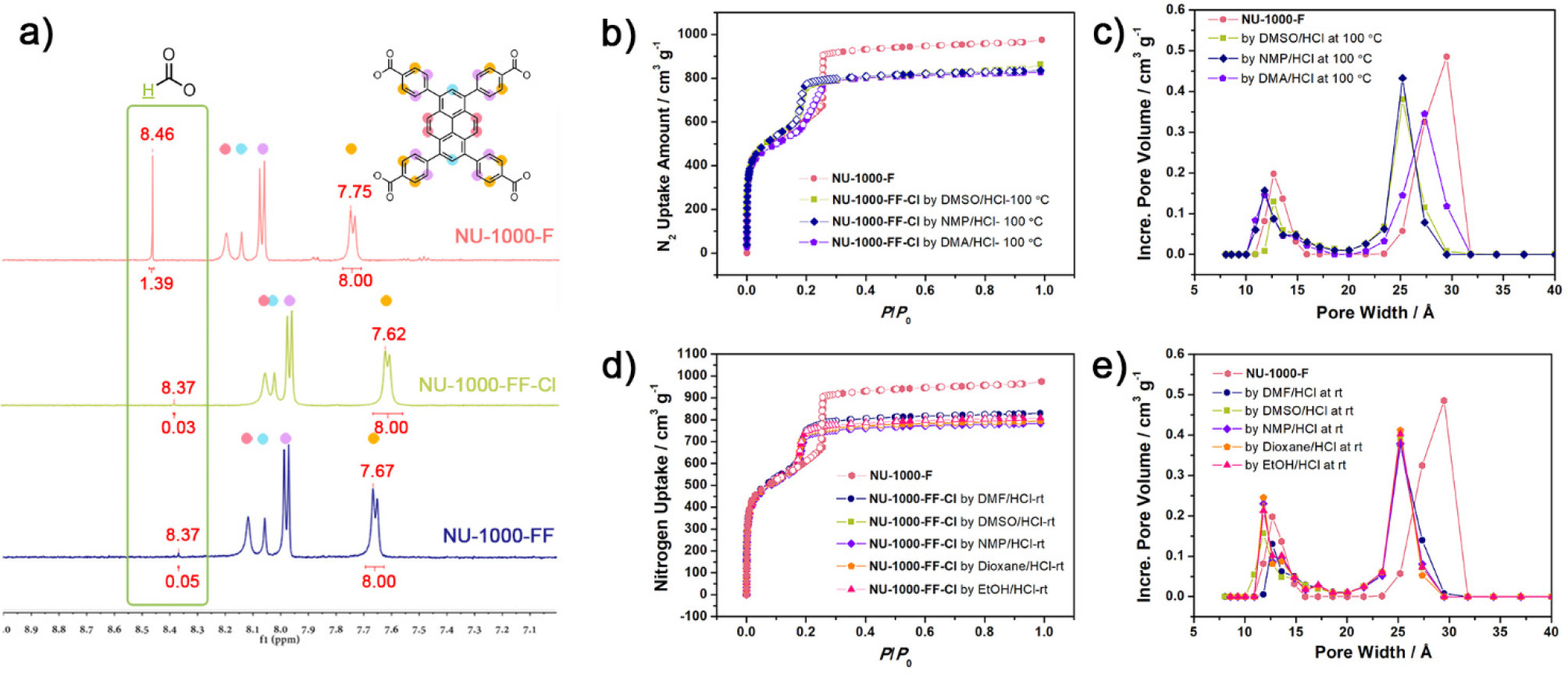
1) BET & pore size: NU-1000-F (2151 m²/g, mesopore 3.1 nm); NU-1000-FF-Cl (2025 m²/g, mesopore 2.5 nm); NU-1000-FF (1803 m²/g).
2) SEM: NU-1000-F (pale yellow), NU-1000-FF-Cl (yellow), NU-1000-FF (golden yellow); no obvious particle size data, but EDS shows Cl/Zr₆=3.4 (FF-Cl) and no Cl (FF).
3) Other tests: ¹H NMR (formate/Zr₆: 2.8 for F, 0.06 for FF-Cl, 0.1 for FF); PXRD (all match simulated, FF/FF-Cl show reversible peak shift); DRIFTS (2745 cm⁻¹ for F, absent for FF/FF-Cl).
3.Application
DMNP hydrolysis (rt, pH 10.5): NU-1000-F (t₁/₂=11 min, TOF=0.01 s⁻¹); NU-1000-FF-Cl (t₁/₂=5 min, TOF=0.03 s⁻¹); NU-1000-FF (0.7 μmol, t₁/₂=3 min, TOF=0.10 s⁻¹); FF retains activity after 6 cycles.
4.Mechanism
- Formate in NU-1000-F bridges Zr(IV) ions, locking node structure and preventing pore contraction; removing formate (FF/FF-Cl) makes aqua ligands labile, enabling reversible pore contraction via aqua ligand loss/uptake.
- More OH/H₂O pairs on FF nodes enhance Lewis acidity, accelerating DMNP hydrolysis by facilitating P=O substitution with aqua ligands.
Outlook:
This research realizes formate-free, fully node-accessible Zr-MOFs, clarifies formate’s interference mechanism, provides a new activation strategy for Zr-MOFs, and promotes their application in catalysis/adsorption.
Node-Accessible Zirconium MOFs
Authors: Zhiyong Lu, Jian Liu, Xuan Zhang, Yijun Liao, Rui Wang, Kun Zhang, Jiafei Lyu, Omar K. Farha, Joseph T. Hupp
DOI: 10.1021/jacs.0c09782
Link: https://pubs.acs.org/doi/10.1021/jacs.0c09782
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

