Home >
News > Evaluation of MOFs for Air Purification and Air Quality Control Applications: Ammonia Removal from Air
Evaluation of MOFs for Air Purification and Air Quality Control Applications: Ammonia Removal from Air
Summary:
The authors from Georgia Institute of Technology, U.S. Army Research, Development, and Engineering Command, and Leidos Inc. developed 10 metal-organic frameworks (MOFs) (including UiO-66 and its 6 variants, ZnBTTB, DMOF-A, DMOF-TM2) with different functional groups and porosities, achieving promising results in the application of ammonia removal from air.
Background:
1. To address the deficiencies of current adsorbents (activated carbons, zeolites) in filtering toxic industrial chemicals (TICs) like ammonia—such as limited developments in synthetic zeolites and difficult control of pore size distributions in activated carbons—previous researchers explored MOFs. Some MOFs (e.g., Cu-BTC, Mg-MOF-74) showed high ammonia capacities but collapsed in humid environments, failing to meet the stability requirement for Chemical Biological Radiological Nuclear (CBRN) filters.
2. The authors in this study proposed using Zr-based UiO-66 MOF (with exceptional thermal, chemical, and mechanical stability) and its functionalized variants, along with two water-stable DMOFs, to remove ammonia from air. They synthesized these materials and tested their ammonia adsorption performance under dry and humid conditions, obtaining insights into the effect of functional groups and porosity on adsorption capacity.
Research Content:
1. Synthesis
-ZnBTTB: Synthesized solvothermally by mixing Zn(NO₃)₂·6H₂O and BTTB ligand in N,N′-diethylformamide (DEF), ethanol, water, and 1 N HCl, heating at 100 °C for 4 days in a Teflon-lined stainless steel reactor; activated by heating at 250 °C for 2 h under vacuum.
-DMOF-A and DMOF-TM2: Synthesized and activated via solvothermal methods as reported in previous work by the authors.
-UiO-66: Synthesized by mixing zirconium(IV) chloride and 1,4-benzenedicarboxylic acid (BDC) in dimethylformamide (DMF), heating at 120 °C for 24 h; activated by heating at 200 °C under vacuum overnight.
-UiO-66-NO₂ and UiO-66-NH₂: Synthesized solvothermally by mixing ZrCl₄ with BDC-NO₂ or BDC-NH₂ ligand in DMF at 120 °C for 24 h; activated by heating at 170 °C (UiO-66-NO₂) and 200 °C (UiO-66-NH₂) under vacuum overnight.
-UiO-66-X (X=–OH, –(OH)₂, –SO₃H, –(COOH)₂): Synthesized solvothermally by mixing ZrOCl₂·8H₂O with corresponding ligands (BDC-OH, BDC-(OH)₂, BDC-SO₃Na, BDC-(COOH)₂) in N,N′-dimethylacetamide (DMA) and formic acid, heating at 150 °C for 24 h; activated by heating at 65 °C under vacuum overnight.
2. Characterizations
1.BET and pore size distribution:
- BET surface areas: ZnBTTB (446 m²/g), DMOF-A (760 m²/g), DMOF-TM2 (1050 m²/g), UiO-66 (1100–1250 m²/g), UiO-66-NH₂ (1096 m²/g), UiO-66-NO₂ (729 m²/g), UiO-66-OH (946 m²/g), UiO-66-(OH)₂ (814 m²/g), UiO-66-SO₃H (323 m²/g), UiO-66-(COOH)₂ (221 m²/g).
- Pore sizes: UiO-66 (~6 Å), UiO-66 variants (<6 Å), ZnBTTB (4.468 Å), DMOF-A (7.5 Å, 3.2 Å), DMOF-TM2 (3.5 Å). Functionalization reduced UiO-66’s pore space.
2.SEM/TEM tests: Not mentioned in the article.
3.Other tests:
- Powder X-ray diffraction (PXRD): Confirmed UiO-66 variants are crystalline and isostructural to parent UiO-66.
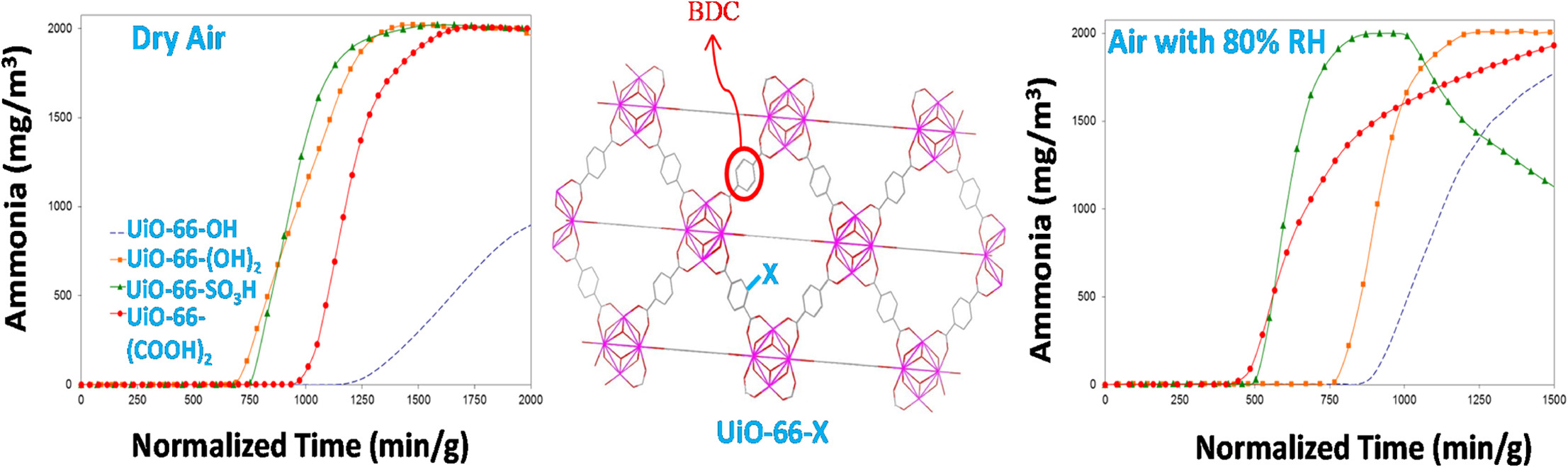
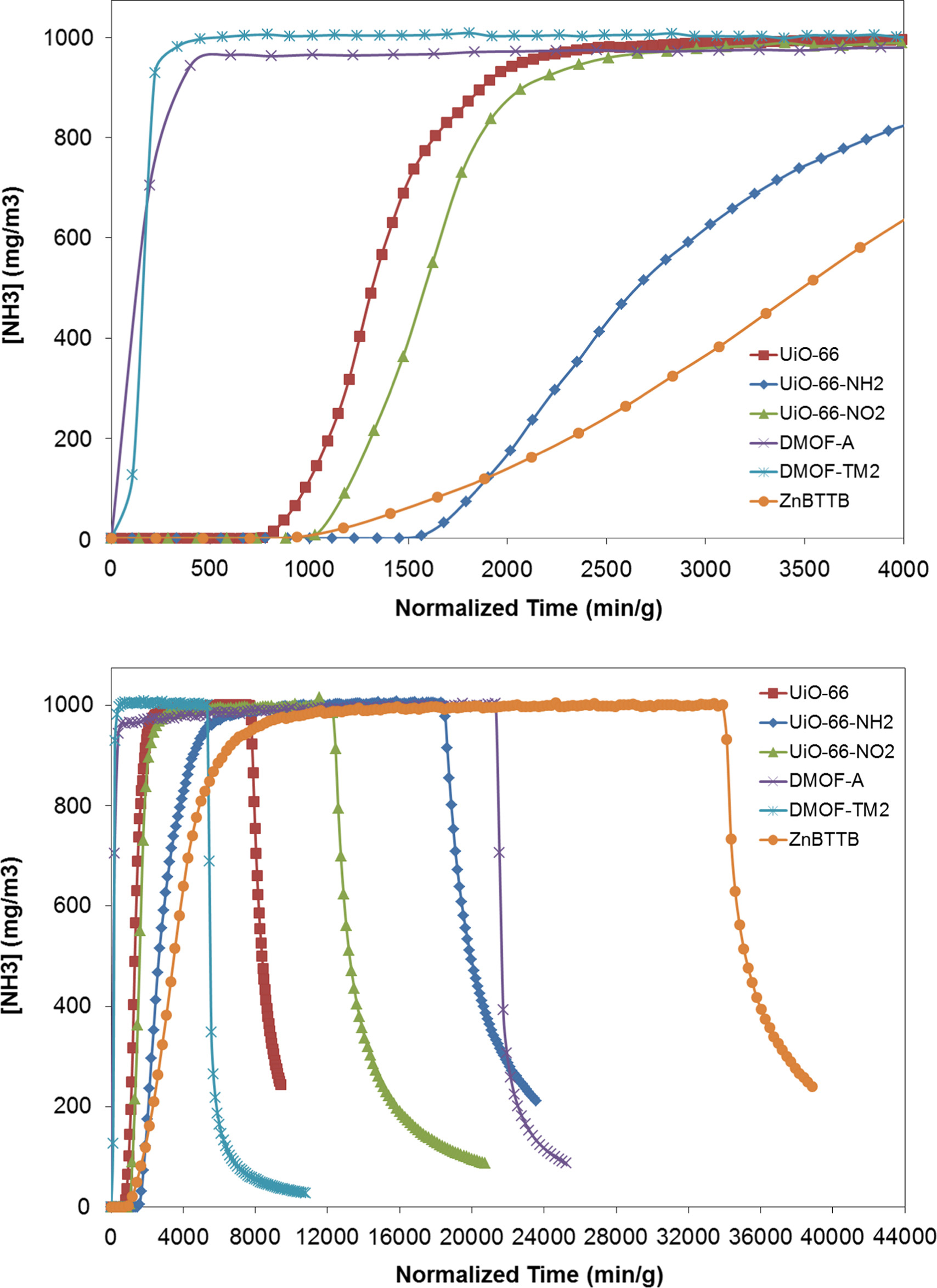
- Ammonia breakthrough measurements: Conducted via a microbreakthrough system under dry (0% RH) and humid (80% RH) conditions at 20 °C, with effluent concentration monitored by a gas chromatograph.
3. Application
The materials were tested for ammonia removal from air. Key results:
-Dry conditions: UiO-66-OH had the highest capacity (~5.69 mmol/g), followed by UiO-66-NH₂ (~3.56 mmol/g); ZnBTTB (~4.59 mmol/g); DMOF-A (~0.48 mmol/g), DMOF-TM2 (~0.15 mmol/g); UiO-66-SO₃H (~2.24 mmol/g), UiO-66-(COOH)₂ (~2.83 mmol/g) had lower capacities.
-Humid conditions: Capacities of functionalized UiO-66 variants decreased (e.g., UiO-66-OH: ~2.77 mmol/g); ZnBTTB, DMOF-A, DMOF-TM2 capacities increased but showed framework decomposition; parent UiO-66 capacity increased (~2.75 mmol/g) due to ammonia solubility in water.
4. Mechanism
-Ammonia adsorption factors: High capacity depends on the interplay of functional groups, surface area, and pore size. –OH groups (less bulky) form stronger H-bonds with ammonia than –NH₂; bulky groups (–SO₃H, –COOH) reduce porosity, lowering capacity.
-Humid environment impact: Water competes with ammonia for active sites on functionalized UiO-66, reducing ammonia adsorption; parent UiO-66’s capacity increase is due to ammonia dissolving in water.
-Desorption behavior: UiO-66-OH and UiO-66-NH₂ show significant desorption after feed termination, indicating ammonia adsorption via weak forces (H-bonding) rather than chemical reaction.
Outlook:
This research successfully synthesized 10 MOFs and identified UiO-66-OH as a promising candidate for ammonia removal—stable in water and with high dry ammonia capacity (~5.69 mmol/g, close to the 6 mmol/g goal). It highlights that balancing water adsorption, ammonia selectivity, and capacity is key for new adsorbents. Future work should explore functionalization of water-stable MOFs with larger pores (e.g., MIL-100, MIL-101) to improve complex functionalization effectiveness.
Evaluation of MOFs for Air Purification and Air Quality Control Applications: Ammonia Removal from Air
Authors: Himanshu Jasuja, Gregory W. Peterson, Jared B. Decoste, Matthew A. Browe, Krista S. Walton
DOI: 10.1016/j.ces.2014.08.050
Link: https://www.sciencedirect.com/science/article/pii/S0009250914004758
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

