Home >
News > Solid-solvent processing of ultrathin, highly loaded mixed-matrix membrane for gas separation
Solid-solvent processing of ultrathin, highly loaded mixed-matrix membrane for gas separation
Summary:
The authors from Nanjing Tech University (China), King Abdullah University of Science and Technology (KAUST, Saudi Arabia), and Suzhou Laboratory (China) developed ultrathin mixed-matrix membranes (MMMs) [e.g., Cu(SiF₆)(pyz)₃@PEG, Cu(SiF₆)(pyz)₃@PVA] with up to 80 vol% MOF loading, <100 nm thickness, and seamless MOF-polymer interface. These MMMs achieved superior H₂-CO₂ separation performance (permeance/selectivity 1–2 orders higher than state-of-the-art membranes) in hydrogen production and precombustion CO₂ capture fields.
Background:
1. To address the demand for efficient gas separation (e.g., H₂ purification, CO₂ capture), previous researchers developed two main membrane types:
Polymeric membranes: Dominant in the market but suffer from inherent permeability-selectivity trade-off due to lack of regular subnanometer channels.
Nanoporous crystalline membranes (zeolites/MOFs): Possess well-defined pores for high permeability/selectivity but face challenges in controlling intergranular defects and large-scale processability.
Traditional MMMs (solution-mixing method): Aim to combine polymer processability and MOF performance, but struggle with interfacial incompatibility (filler agglomeration, defects) at high filler loading (>30–40 vol%) and cannot form ultrathin selective layers (due to casting solution penetration into substrates).
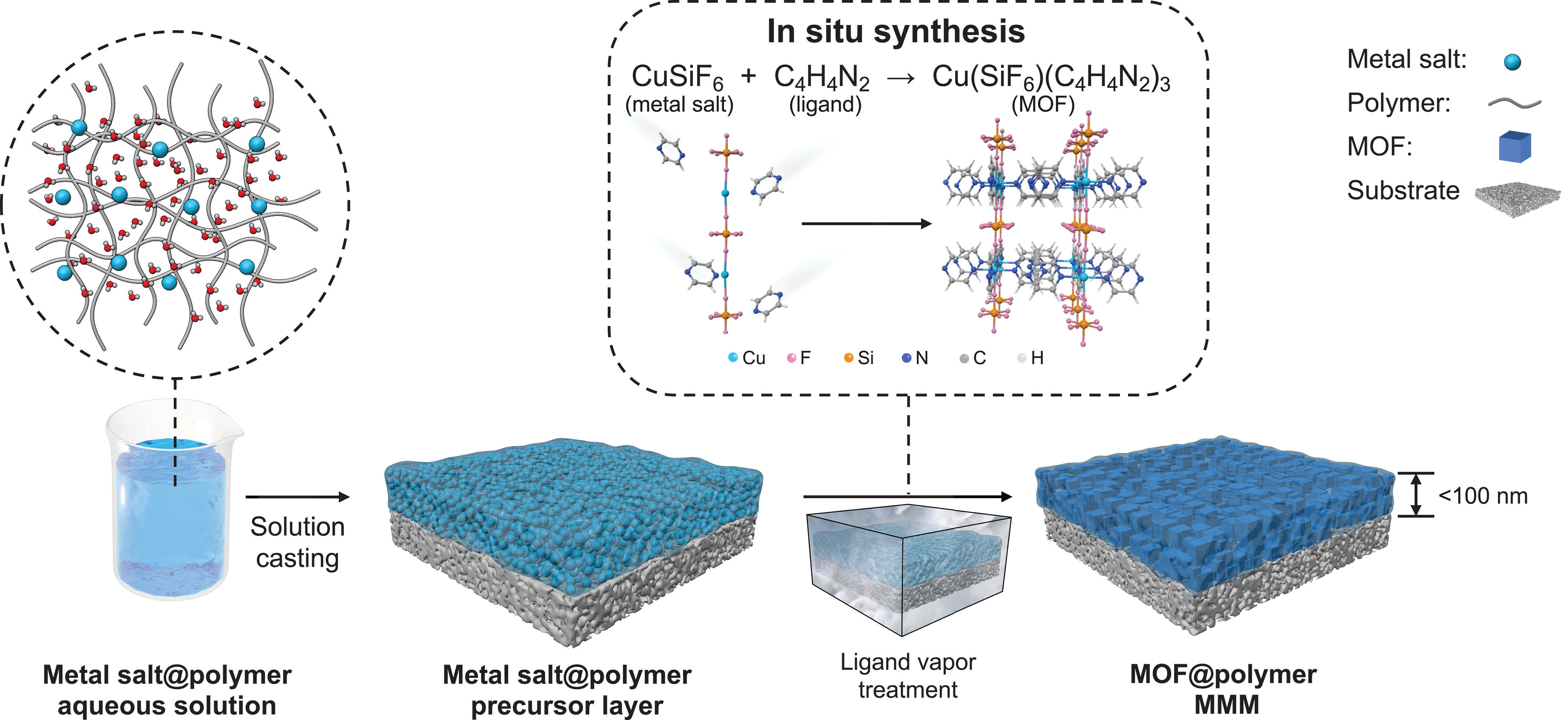
2. The authors proposed an innovative solid-solvent processing (SSP) strategy: Using polymers (PEG/PVA) as "solid solvents" to dissolve/immobilize metal salts, form ultrathin precursors, and enable in-situ MOF conversion via ligand vapor. This solves high-loading agglomeration and ultrathinning issues, yielding MMMs with interconnected MOF channels and seamless interfaces.
Research Content:
1. Synthesis
Core SSP Workflow (Fig. 1):
1. Precursor Preparation: Dissolve metal salts (e.g., CuSiF₆, Zn(NO₃)₂, NiSiF₆) and polymers (PEG/PVA) in aqueous solution to form uniform metal salt@polymer solutions.
2. Ultrathin Precursor Coating: Spin-coat the solution onto porous PAN substrates (pore size ~20 nm); evaporate solvent to form defect-free metal salt@polymer precursor layers (thickness tunable via spin-coating cycles: 50–90 nm with 10–40 cycles).
3. In-situ MOF Conversion: Place precursor and ligand (e.g., pyrazine (pyz), 4,4’-bipyridine (bpy), 2-methylimidazole) in a closed reactor; heat to vaporize ligand, which diffuses into the precursor and reacts with metal salts to form MOF@polymer MMMs.
Typical MMM Synthesis Examples:
- Cu(SiF₆)(pyz)₃@PEG: Use PEG (Mᵥ=10,000 g/mol) and CuSiF₆ (mass ratio 1:5); react with pyz vapor at elevated temperature.
- Cu(SiF₆)(pyz)₃@PVA: Use PVA (Mᵥ=75,000 g/mol) and CuSiF₆ (mass ratio 1:2.5); optimal for high-temperature applications.
- ZIF-L@PEG: Convert Zn(NO₃)₂@PEG precursor via 2-methylimidazole vapor.
2. Characterizations
1) BET & Pore Size:
- MOF components (e.g., Cu(SiF₆)(pyz)₃) have well-defined subnanometer channels (window aperture 2.2×2.5 Å) confirmed by positron annihilation spectroscopy (Fig. 3B: right shift of positron lifetime indicates increased subnanometer cavities after MOF conversion).
- MMMs with >50 vol% MOF form interconnected MOF channels (dominant for gas transport), as verified by transport resistance models (Fig. 3D–E).
2) SEM/TEM & Particle Size:
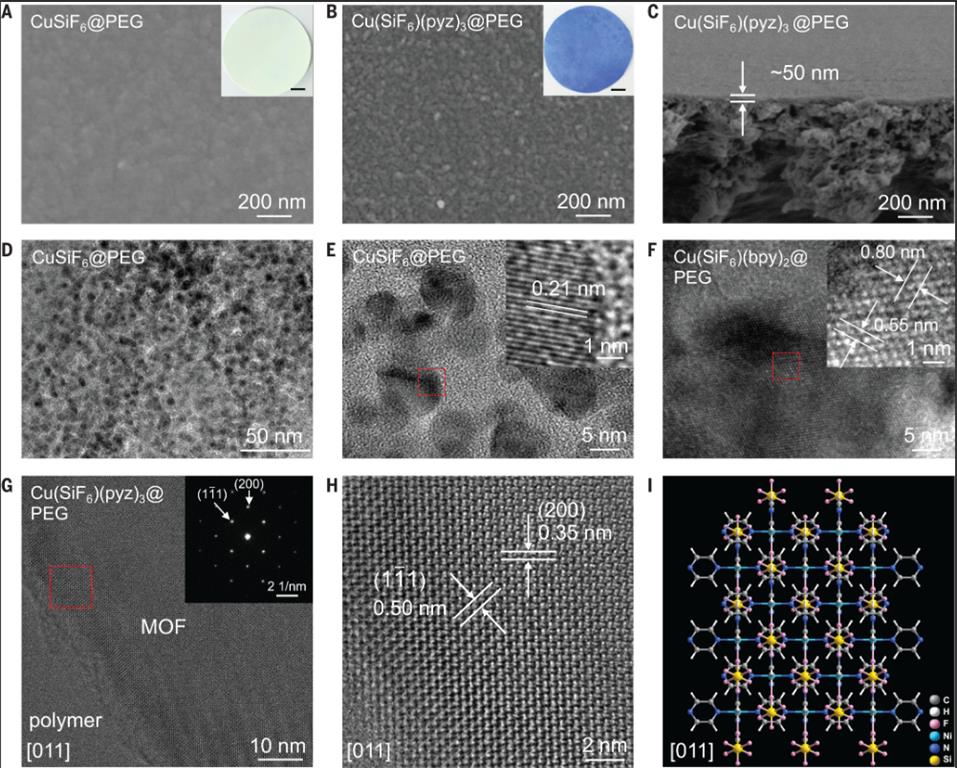
SEM (Fig. 2A–C): Precursor layers (CuSiF₆@PEG) show smooth, defect-free surfaces; after MOF conversion, granular protrusions appear, and cross-sections confirm 50–90 nm thickness.
TEM/EDX (Fig. 2D–F): CuSiF₆ is uniformly dispersed in PEG as 5–10 nm nanoparticles (d-spacing 0.21 nm, nonporous); after conversion, scattered nanoparticles are replaced by ordered Cu(SiF₆)(bpy)₂ crystals (d-spacings 0.80 nm (002) and 0.55 nm (020)).
Ultralow-dose HRTEM (Fig. 2G–I): Seamless adhesion between amorphous polymer and crystalline MOF; Cu(SiF₆)(pyz)₃ shows lattice fringes (0.50 nm (111), 0.35 nm (200)), matching [011]-projected structure models.
3) Other Characterizations:
XRD (Fig. 3A): Precursor shows CuSiF₆ peaks; after conversion, peaks match simulated Cu(SiF₆)(pyz)₃, confirming complete MOF formation.
Thermogravimetry (TGA): Calculated MOF loading up to 80.4 vol% (Cu(SiF₆)(pyz)₃@PEG) and 59.6 vol% (Cu(SiF₆)(pyz)₃@PVA); PVA-based MMMs have higher Tg (~80°C) than PEG-based (~60°C) for high-temperature stability.
XPS/IR: Confirm presence of ligand (pyz/bpy) and structural conversion (e.g., N 1s peaks from pyz, Si-F stretching in 800–1200 cm⁻¹).
Water Adsorption: Ni(NbOF₅)(pyz)₃@PVA MMM maintains stable H₂ permeance (1118 GPU) and H₂-CO₂ selectivity (32.7) under 3 vol% water vapor (120°C, 1 MPa), indicating water stability.
Additional Performance Highlights:
- Surpasses the Robeson upper bound for H₂-CO₂ separation (Fig. 3E); outperforms state-of-the-art membranes (e.g., PBI, TR polymers, MOF-based MMMs) by 1–2 orders in permeance/selectivity.
- Flexibility: Cu(SiF₆)(pyz)₃@PEG MMM (80.4 vol% loading) maintains performance after curling (Fig. 4D); no cracks/defects observed via SEM.
- Scalability: Successfully fabricated PAN hollow-fiber MMMs (~100 nm thickness) and large-area flat-sheet MMMs; 12 sampled portions show stable separation performance (Fig. 4H).
4. Mechanism
1. Ultrathin/High-Loading Mechanism:
- Polymers (PEG/PVA) act as "solid solvents" with high cosolubility for metal salts, enabling uniform dispersion (no agglomeration) and ultrathin coating (avoids substrate penetration via controlled polymer molecular weight: PEG Mᵥ=10,000–70,000 g/mol).
- In-situ MOF conversion occurs within the polymer matrix; flexible polymer segments dynamically adhere to MOF crystals, forming seamless interfaces and compensating for intergranular defects.
2. Gas Separation Mechanism:
Interconnected MOF Channels: At >50 vol% MOF loading, MOFs form a continuous phase (Fig. 3D); gas transport is dominated by MOF pores (not polymer), achieving performance close to pure MOF membranes.
Size-Sieving Effect (DFT Verification): Cu(SiF₆)(pyz)₃ pores (2.2×2.5 Å) match H₂ kinetic diameter (2.89 Å) better than CO₂ (3.3 Å). DFT calculations (CI-NEB method) show CO₂ needs higher diffusion energy barrier (7.61 kcal/mol) than H₂ (6.46 kcal/mol); larger van der Waals surface overlap between MOF and CO₂ further hinders CO₂ transport (Fig. 3F).
Low Water Affinity: MOFs with Ni(II)-pyrazine/(NbOF₅)²⁻ pillars reduce water adsorption; polymer matrix isolates MOFs from moisture, maintaining stability in humid syngas.
Outlook:
This research achieves three key breakthroughs:
1. Method Innovation: The SSP strategy solves long-standing issues of traditional MMMs (high-loading agglomeration, ultrathinning difficulty) by using polymers as solid solvents, enabling MOF loading up to 80 vol% and <100 nm thickness.
2. Performance Leap: The MMMs exhibit superior H₂-CO₂ separation (permeance 3640 GPU, selectivity 76.1) that surpasses state-of-the-art membranes, bridging the gap between MOF performance and polymer processability.
3. Universality & Scalability: SSP works for diverse MOFs (SIFSIX-series, ZIFs) and polymers (PEG/PVA); successfully scaled to hollow-fiber and large-area flat-sheet membranes, facilitating industrial application.
The strategy not only provides a new platform for high-performance gas separation membranes but also paves the way for translating nanomaterials into functional thin films (e.g., sensors, catalysis). Future work could focus on optimizing polymer-MOF compatibility and expanding to other separation systems (e.g., C₂H₄-C₂H₆, CH₄-CO₂).
Solid-solvent processing of ultrathin, highly loaded mixed-matrix membrane for gas separation
Authors: Guining Chen, Cailing Chen, Yanan Guo, Zhenyu Chu, Yang Pan, Guozhen Liu, Gongping Liu, Yu Han, Wanqin Jin, Nanping Xu
DOI: 10.1126/science.adi1545
Link: https://www.science.org/doi/10.1126/science.adi1545
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

