Home >
News > Highly Selective Carbon Dioxide Uptake by [Cu(bpy-n)₂(SiF₆)] (bpy-1=4,4'-Bipyridine; bpy-2=1,2-Bis(4-pyridyl)ethene)
Highly Selective Carbon Dioxide Uptake by [Cu(bpy-n)₂(SiF₆)] (bpy-1=4,4'-Bipyridine; bpy-2=1,2-Bis(4-pyridyl)ethene)
Summary:
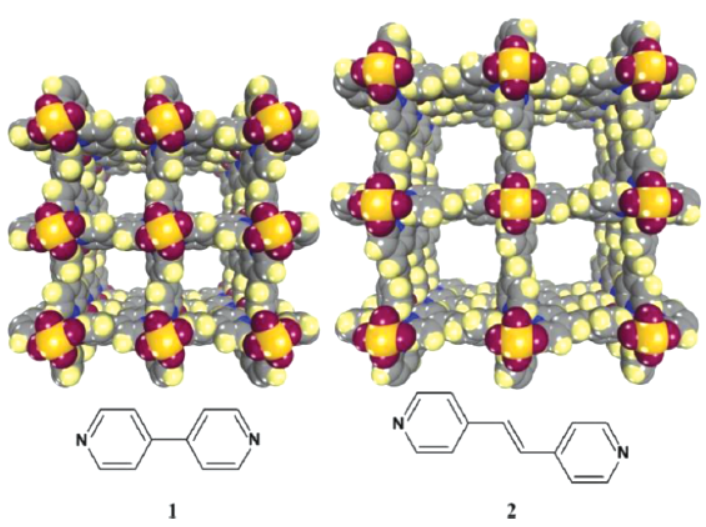
The authors from University of South Florida, Northwestern University, and Pacific Northwest National Laboratory (USA) developed two porous coordination polymers (PCPs) — [Cu(bpy-1)₂(SiF₆)] (bpy-1=4,4'-bipyridine) and [Cu(bpy-2)₂(SiF₆)] (bpy-2=1,2-bis(4-pyridyl)ethene) — with high surface area, tunable pore size, and no open metal sites. They achieved excellent selective CO₂ uptake (surpassing most PCPs/ZIFs) in the application field of carbon capture and methane purification.
Background:
1. To address the urgent need for efficient CO₂ capture (e.g., greenhouse gas control, methane purification), previous researchers developed metal-organic materials (MOMs) like Mg/DOBDC (MOF-74 analog) and zeolitic imidazolate frameworks (ZIFs). Mg/DOBDC has high CO₂ selectivity but relies on open metal sites (causing high regeneration energy and water affinity); ZIFs have low CO₂/CH₄ selectivity, low surface area, and poor ambient uptake. Existing PCPs with open metal sites also suffer from reduced CO₂ uptake after water exposure.
2. The authors proposed an innovative strategy: using PCPs of the formula [Cu(bpy-n)₂(SiF₆)] with saturated Cu centers (no open metal sites) and tunable pore size (via bpy-n linker length). This avoids chemisorption-related drawbacks, retains high surface area, and achieves high CO₂ selectivity through physisorption, outperforming ZIFs and approaching Mg/DOBDC.
Research Content:
1. Synthesis
-[Cu(bpy-1)₂(SiF₆)]: Prepared by layering method: dissolve CuSiF₆ (0.05 mmol, 10.3 mg) in 2 mL 50:50 EtOH:H₂O, then layer onto a solution of bpy-1 (0.1 mmol, 15.6 mg) in 2 mL CHCl₃. Purple cubic crystals form after 1 week.
-[Cu(bpy-2)₂(SiF₆)]: Prepared by solvothermal method: dissolve CuSiF₆ (0.1 mmol, 20.6 mg) in 8 mL H₂O and bpy-2 (0.2 mmol, 36.4 mg) in 8 mL MeOH; add 250 μL nitrobenzene, heat at 85 °C for 24 h, and cool to obtain purple cubic crystals.
-Activation: Both materials undergo MeOH solvent exchange (3 times/day for 3 days) and degassing (16 h at 25 °C under high vacuum, 1×10⁻⁶ bar) before gas sorption tests.
2. Characterizations
1)BET and pore size distribution:
- [Cu(bpy-1)₂(SiF₆)]: N₂ uptake (77 K) = 425 cm³/g; BET surface area = 1468 m²/g, Langmuir surface area = 1651 m²/g; effective pore window size (van der Waals correction) ≈ 8 Å.
- [Cu(bpy-2)₂(SiF₆)]: N₂ uptake (77 K) = 740 cm³/g; BET surface area = 2718 m²/g, Langmuir surface area = 3118 m²/g; effective pore window size ≈ 10.6 Å.
- Both have narrow pore size distribution (square channels parallel to c-axis, controlled by bpy-n linkers) confirmed by X-ray diffraction.
2)X-ray diffraction (single-crystal/powder):
- Single-crystal XRD: Both are tetragonal (Table S2): [Cu(bpy-1)₂(SiF₆)] (space group P4/mmm, a=b=11.045(8) Å, c=8.163(6) Å, V=995.8(12) ų, Z=1); [Cu(bpy-2)₂(SiF₆)] (space group P4/nmm, a=b=18.8471(11) Å, c=7.9975(5) Å, V=2840.8(3) ų, Z=2). Pyridyl moieties are disordered over two positions.
- Powder XRD (Figures S1-S2): Pre- and post-activation patterns match calculated ones, confirming phase purity and framework stability after activation.
3)Other tests:
-Thermogravimetric Analysis (TGA, Figures S3-S4): [Cu(bpy-1)₂(SiF₆)] shows weight losses of 23.47%, 29.07%, 33.57% (solvent loss); [Cu(bpy-2)₂(SiF₆)] has losses of 24.45% and 57.93%, indicating thermal stability up to ~300 °C.
-Infrared Spectroscopy (IR, Figure S5): Characteristic peaks confirm presence of bpy-n ligands and SiF₆²⁻ (e.g., 800-1200 cm⁻¹ for Si-F stretching).
-Water Adsorption (Figure S8): Low uptake at P/P⁰=1: [Cu(bpy-1)₂(SiF₆)] ≈20.5 wt%, [Cu(bpy-2)₂(SiF₆)]≈19 wt% (far lower than HKUST-1 (52%) and MIL-101 (135%)), confirming low water affinity.
-CO₂ Heat of Adsorption (Qst, Figure S10): [Cu(bpy-1)₂(SiF₆)] ≈27 kJ/mol, [Cu(bpy-2)₂(SiF₆)]≈21 kJ/mol (physisorption range, lower than Mg/DOBDC (47 kJ/mol) but comparable to HKUST-1 (30 kJ/mol)).
3. Application
CO₂/CH₄/N₂ Selective Adsorption (298 K, 1 atm):
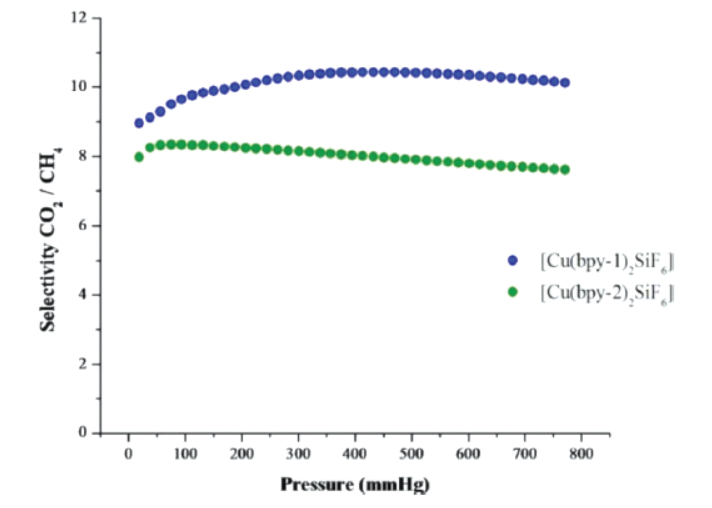
-[Cu(bpy-1)₂(SiF₆)]: CO₂ uptake =23.1 wt% (double Zeolite-13X, approaching Mg/DOBDC); CH₄ uptake=0.83 wt%, N₂ uptake negligible; CO₂/CH₄ selectivity=10.5:1 (highest among PCPs without open metal sites).
-[Cu(bpy-2)₂(SiF₆)]: CO₂ uptake=12.1 wt% (half of bpy-1 analog but still high); CH₄ uptake=0.71 wt%; CO₂/CH₄ selectivity=8:1 (maintained despite double surface area).
-IAST Calculations (Figure 3): Selectivities remain consistent up to 1 atm, matching Henry law constant ratios (linear single-component isotherms, Figure S7/S9).
-Reversibility: N₂ and CO₂ isotherms (Figures S6/S9) show overlapping adsorb-desorb curves, confirming framework stability during cycles.
4. Mechanism
-Low Water Affinity: Saturated Cu²⁺ centers (no open metal sites) avoid strong coordination with H₂O, unlike MOMs with unsaturated metals (e.g., Mg/DOBDC), thus maintaining CO₂ uptake in humid environments.
-CO₂ Selective Physisorption:
1. Tunable pore size (8 Å for bpy-1, 10.6 Å for bpy-2) matches CO₂ kinetic diameter (3.3 Å) better than CH₄ (3.8 Å) and N₂ (3.6 Å), enhancing size-based selectivity.
2. High surface area provides abundant adsorption sites; SiF₆²⁻ pillars form non-interpenetrating frameworks (no pore blockage), maximizing accessible pores.
3. Moderate Qst (21-27 kJ/mol) promotes CO₂ adsorption but allows low-energy regeneration (physisorption), avoiding chemisorption drawbacks.
-Isoreticular Structure Advantage: Bpy-n linker length controls pore size, enabling optimization for different gas separation scenarios (e.g., bpy-1 for high CO₂ uptake, bpy-2 for larger pore applications).
Outlook:
This research successfully developed two [Cu(bpy-n)₂(SiF₆)] PCPs that balance high CO₂ selectivity, low water affinity, and low regeneration energy (via physisorption). Key achievements include: 1) Highest CO₂/CH₄ selectivity (10.5:1) for PCPs without open metal sites; 2) Tunable pore size via linker design, expanding application flexibility; 3) Low-cost synthesis from commercial reagents, facilitating scale-up. The work establishes a new PCP platform for carbon capture, addresses limitations of open-metal-site MOMs, and guides future design of physisorption-based selective adsorbents (e.g., optimizing SiF₆²⁻ interactions via modeling).
Highly Selective Carbon Dioxide Uptake by [Cu(bpy-n)₂(SiF₆)] (bpy-1=4,4'-Bipyridine; bpy-2=1,2-Bis(4-pyridyl)ethene)
Authors: Stephen D. Burd, Shengqian Ma, Jason A. Perman, Benjamin J. Sikora, Randall Q. Snurr, Praveen K. Thallapally, Jian Tian, Lukasz Wojtas, Michael J. Zaworotko
DOI: 10.1021/ja211340t
Link: https://pubs.acs.org/doi/10.1021/ja211340t
The above review is for academic progress sharing. For any errors or copyright issues, please contact us for correction or removal.

